Food Safety Training and Certification

Institute of Good Manufacturing Practices India
DISTINGUISH YOURSELF AS AN FSSAI CERTIFIED FOOD HANDLER
By opting courses at IGMPI (FSSAI approved training Institute)
As per FSSAI requirements, each Food Business Operator needs to have trained & certified person in their business premises to ensure food safety. To implement this regulation FSSAI has approved Training partners with an objective to ensure safe food everywhere. The curriculum and content of training has been created centrally.
Topic:
FSSAI FoSTaC Food Safety Supervisor Training




Who Should Attend:
All employees who are involved in the Food sector and aspiring food professionals. This course is relevant to all those involved in any aspect of food handling, through the supply chain, retail, and food preparation. It is ideal also for those working in professional kitchens, canteens, hotels, manufacturing as well as food producers.
Training Benefits:
-
Certificate from FSSAI, after successful completion of the training.
-
Understanding basic concepts of food safety & hygiene.
Training Details:
Upcoming Training Schedule
Duration: 1 day
Duration: Special Training Milk & Milk Products 1 nd half day
Timings : 9:00 AM to 5:00 PM
The training schedule will be finalised mutually depending upon the number of participants/nominations.
Note: The FOSTAC Training may also be conducted at your company premises on your request.
Fees Details :
FSSAI FosTac Training Fee:
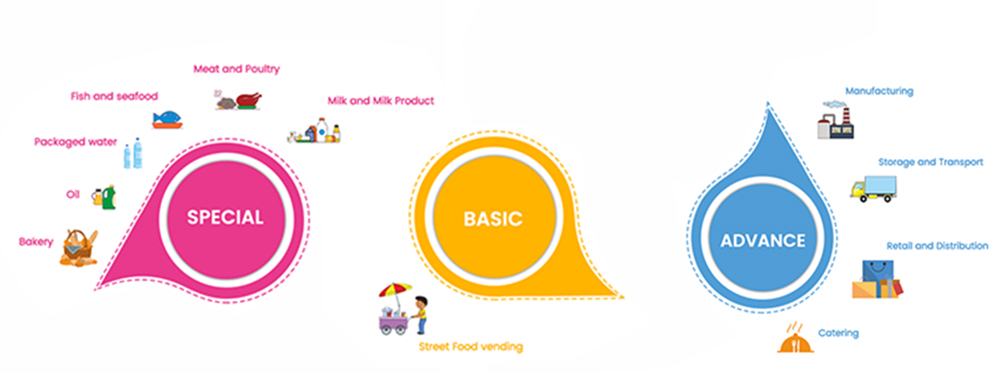
1. Advanced Training- The duration of each course is 8 hours. The fee for the courses are Rs.2000/-
Courses are: -
- Manufacturing / Processing
- Catering
- Storage & Transport
- Retail & Distribution
2. Special Training- The duration of the special course is 8-12 hours (1-2 days). The Fee for the course is Rs.2400/- .
Courses are: -
- Milk & Milk Products
- Animal Meat & Meat Products
- Poultry Meat & Meat Products
- Fish & Fish Products
- Packaged Water & Water Based Beverages
- Bakery and Confectionery (level-1)
- Bakery and Confectionery (level-2)
- Edible Oil & Fat
- Health Supplements & Nutraceuticals
3. Basic Training - The duration of each course is 8 hours. The fee for each course is Rs.1200/.
Courses are: -
- Manufacturing / Processing & Covid (Level-1)
- Catering & Covid (Level-1)
- Storage & Transport (Level-1)
- Retail & Distribution (Level-1)
- Street Food Vending & Covid (Level-1)
Completed Training Details: Other PG Diploma and Certification Programmes: View here
DATE:
1: 8th November 2017 / (Completed)
2: 16th November 2017 / (Completed)
3: 29th November 2017 / (Completed)
4: 19th December 2017 / (Completed)
5: 22nd December 2017 / (Completed)
6: 22nd February 2018 / (Completed)
7: 23nd February 2018 / (Completed)
8: 20th March 2018 / (Completed)
9: 27th July 2018 / (Completed)
10: 15th September 2018 / (Completed)
11: 20th September 2018 / (Completed)
12: 21st December 2018 / (Completed)
13: 21st January 2019 / (Completed)
14: 27th February 2019 / (Completed)
15: 8th June 2019 / (Completed)
16: 2nd August 2019 / (Completed)
17: 16th November 2019 / (Completed)
18: 29th February 2020 / (Completed)
19: 25th April 2020 / (Completed)
20: 26th April 2020 / (Completed)
21: 24th January 2021 / (Completed)
22: 14th March 2021 / (Completed)
23: 15th May 2022 / (Completed)
24: 26th June 2022 / (Completed)
25: 3rd July 2022 / (Completed)
26: 17th September 2022 / (Completed)
27: 24th September 2022 / (Completed)
IGMPI Live Interactive Classes: View here
IGMPI is Conferred with QCI - D. L. Shah Quality Award
ASSOCHAM Services Excellence Award 2017


-copy.jpg)