Trusted by organization & traning participants in over 45 countries
Traning | Certification | Education | Research
Prospectus
Academic Year 2025-26 (Session January-February, 2026)
Diploma in Pharmaceutical Quality Assurance and Quality Control (DPQAQC 12 months), Diploma in Pharmaceutical Management (DPM 12 months), Diploma in Pharmaceutical Packaging (DPP 12 months), Diploma in Cosmetic Technology (DCT 12 months), Diploma in Herbal Cosmetics (DHC 12 months), Diploma in Environment Health and Safety (DEHS 12 months), Diploma in Medical Coding (DMC 12 months), Diploma in Medical Record Technology (DMRT 12 months), Diploma in Naturopathy and Yogic Science (DNYS 12 months), Diploma in Ayurveda Practices (DAP 12 months), Diploma in Hospital Management (DHM 12 months), Diploma in Health and Sanitation (DHS 12 months), Diploma in Industrial Hygiene (DIH 12 months)
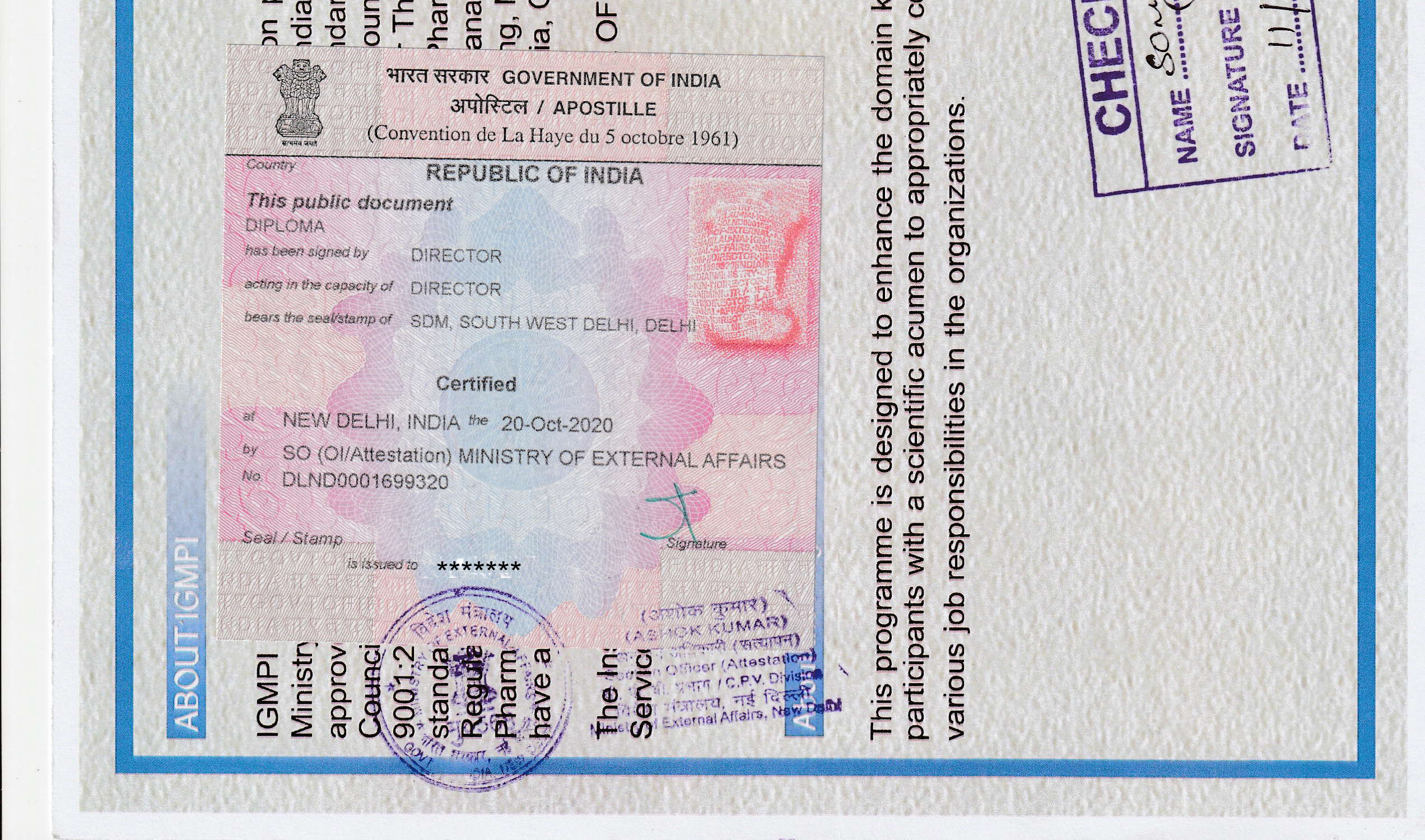
igmpi.ac.inAbout IGMPI
The Institute of Good Manufacturing Practices India, an autonomous Institute recognised by Ministry of Commerce & Industry, accredited Vocational Institution of Ministry of Education, Government of India and approved by Food Safety and Standards Authority of India (FSSAI), presents a unique, friendly and interactive platform to get rid of all your GMP compliance related issues. GMP—an essential element of industries like pharmaceutical, cosmetic, Ayurveda, biotech, homeopathic, medical device and food manufacturing and sustainability services—in itself is the most dynamic part which witnesses frequent changes in terms of new rules being added and old ones being renewed. Thus, keeping oneself updated with current GMPs is essential to remain aligned with evolving industry requirement and standards.
Our team comprises knowledgeable professionals from diverse sectors such as Pharma, Healthcare, Food, Nutraceutical, and Nutrition industries, pooling together their expertise, know-how, and experiences to create this GMP guide. IGMPI is moving hand in hand with technology advances and has gained recognition as a stronger and better education and training platform provider for professionals and students in the areas of GMP, Quality Assurance and Control, Pharma, Food & Nutrition, Environment and Healthcare Regulatory Affairs, Clinical Research, Pharmaceutical IPR, Good Laboratory Practice and Product Management. Keeping pace with the digital era, IGMPI has also expanded into the domains of Geoinformatics, Remote Sensing, Cybersecurity, Information Security, and Network Security, preparing learners to address the growing need for secure and resilient information systems as well as advanced geospatial solutions.
Our board of governors and specialists have collated their acumen and are offering state-of-the-art courses which include GMP training, Quality Assurance and Control, Pharma and healthcare Regulatory Affairs, Clinical Research, Pharmaceutical IPR, Environment Social Governance (ESG), Good Laboratory Practice, Information Security Compliance and Remote Sensing. These are delivered in the form of formal classroom studies, online/interactive programmes, online seminars, and onsite training programmes, along with insights into global industry practices. In short, a round-the-clock service is provided for any information in these areas required by anybody from around the country and abroad.
Based on high standards of quality, the training programmes in Pharma, Healthcare and Food GMP, Quality Assurance and Quality Control, Regulatory Affairs, IPR, Pharma Product Management, Public Health, Hospital Management, Clinical Research, Pharmacovigilance, Medical Writing, Medical Coding, Nanotechnology, Drug Design and Discovery, Food QA&QC have been approved by the Quality Council of India, which is an autonomous body and an accreditation authority for education & vocational training providers under the Ministry of Commerce & Industry, Government of India. IGMPI is duly licensed and certified by Bureau of Indian Standards (BIS) under Bureau of Indian Standards (Conformity Assessment) Regulations 2018.
Accreditation and Awards
The Institute of Good Manufacturing Practices India, an autonomous Institute recognised by the Ministry of Commerce & Industry, Government of India is duly licensed and certified by Bureau of Indian Standards (BIS) under Bureau of Indian Standards (Conformity Assessment) Regulations 2018 (Active license number: ERO/EOMSM/L-8000027) for offering education and training programmes in the sectors of Pharmaceutical, Food & Nutrition, Healthcare, Environmental Health & Safety, Environmental Social and Governance (ESG), Psychology, Medical Devices, Cosmetics, Remote Sensing, Health Insurance, Product & Marketing Management, Cybersecurity, Fire Safety, and Renewable Energy in the regular Diploma (full or part-time) and online modes.


IGMPI is an accredited Vocational Institution (AVI no. 710367) of the Ministry of Education, Government of India. Institute of Good Manufacturing Practices India is Management System certified under NABCB accreditation. IGMPI is also approved by Food and Life Sciences Sector Skill Councils recognized by the National Council of Vocational Education and Training (NCVET).

The Post Graduate and Executive Diploma programmes of IGMPI in Good Manufacturing Practices, Regulatory Affairs, Intellectual Property Rights, Quality Assurance and Quality Control, Public Health, Nanotechnology, Hospital Management, Product Management, Sales and Marketing Management, Clinical Research, Medical Writing, Drug Discovery and Development, Pharmacovigilance, Medical Coding have been duly assessed and approved by Quality Council of India, Government of India based on fulfillment of QCI's following criteria:
1. Course Content
2. Course Design
3. Course Material
4. Instructors
5. Class size & Attendance
6. Facilities
7. Evaluation of Students
8. Written Examination
9. Certificate
IGMPI is also approved by Food Safety and Standards Authority of India (FSSAI) (FSSAI ID: TPINS18). IGMPI® is licensed by Department of Food Safety & Drug Administration under the Drugs and Cosmetics Act, 1940 and registered under Food Safety and Standards Act 2006.
IGMPI is also approved by Sector Skills Council under National Skill Development Corporation (NSDC) setup by Ministry of Skill Development & Entrepreneurship, Govt of India (TC ID:TC342267).
IGMPI has been conferred with the prestigious "BUREAU OF INDIAN STANDARDS (BIS) AWARD OF HONOUR 2023". QUALITY COUNCIL OF INDIA (QCI) has also conferred IGMPI with D.L. SHAH NATIONAL QUALITY AWARD, Certificate of Merit & ASSOCHAM has conferred IGMPI with the Services Excellence Award based on excellence of its services to the students and training participants.
IGMPI's Membership with IPA
IGMPI is a Lifetime Institutional member of Indian Pharmaceutical Association (IPA).

Bureau of Indian Standards (BIS)
Bureau of Indian Standards (BIS) came into existence through an act of Parliament in 1987. BIS is the National Standard Body of India established under the BIS Act 2016 for the harmonious development of the activities of standardization, marking and quality certification of goods and for matters connected therewith or incidental thereto. The Bureau is a Body consisting of 25 members representing both Central and State governments, Members of Parliament, industry, scientific and research institutions, consumer organizations and professional bodies; with Union Minister of Consumer Affairs, Food and Public Distribution as its President and with Minister of State for Consumer Affairs, Food and Public Distribution as its Vice-President.
Quality Council of India (QCI)
Quality Council of India is set up by the Government of India to establish and operate national accreditation structure and promote quality through National Quality Campaign. QCI is registered as a non-profit society with its own Memorandum of Association. QCI is governed by a Council of 38 members and Chairman of QCI is appointed by the Prime Minister on recommendation of the industry to the government. The Department of Industrial Policy & Promotion, Ministry of Commerce & Industry, is the nodal ministry for QCI.
International Recognition
For providing its education and training services to overseas students, IGMPI is registered with the Directorate General of Foreign Trade, Government of India and our Export Import Code is AADCI7680Q.
IGMPI is an Institutional Member of the International Society for Quality in Health Care

Bureau of Indian Standards (BIS) is a member of International Organization for Standardization (ISO) and through the Indian National Committee (INC) which is a member of International Electrotechnical Commission (IEC). BIS is also a member of regional standards bodies like Pacific Area Standards Congress (PASC) and South Asian Regional Standards Organization (SARSO). India started taking part in IEC from 1911 and subsequently the then Indian Standards Institution (now BIS) took over the responsibility of Indian National Committee of IEC(INC-IEC) in 1949. Since then the INC-IEC is actively participating in the activities of the IEC both at the policy level and technical work and carrying out the responsibilities as member body of IEC Council. India is a member in Standards Management Board (SMB) of IEC since 2015.BIS has also signed Bilateral Cooperation Agreements (BCA)/Mutual Recognition Agreements (MRA) with the National Standards Bodies of several countries like Afghanistan, Bangladesh, Belarus, Egypt, European Union , Germany, Ghana, Greece, Indonesia, Iran, Japan, Jordon, Kenya, Kyrgyzstan, Mali, Mauritius, Nigeria, Russia, Saudi Arabia , Slovakia, Slovenia, Suriname, USA, UAE, Uzbekistan, Viet Nam, Bhutan, Brazil, Israel, Nepal, Pakistan and Sri Lanka.
National Accreditation Board for Certification Bodies (NABCB), Quality Council of India is a member of International Accreditation Forum (IAF) & Pacific Accreditation Cooperation (PAC) as well as signatory to its MLAs for Quality Management Systems, Environmental Management Systems and Product Certification. NABCB is also a Full Member of International Laboratory Accreditation Cooperation (ILAC) & Asia Pacific Laboratory Accreditation Cooperation (APLAC) as well as signatory to its MRAs for Inspection.
Diploma in Pharmaceutical Quality Assurance and Quality Control (DPQAQC 12 months), Diploma in Pharmaceutical Management (DPM 12 months), Diploma in Pharmaceutical Packaging (DPP 12 months), Diploma in Cosmetic Technology (DCT 12 months), Diploma in Herbal Cosmetics (DHC 12 months), Diploma in Environment Health and Safety (DEHS 12 months), Diploma in Medical Coding (DMC 12 months), Diploma in Medical Record Technology (DMRT 12 months), Diploma in Naturopathy and Yogic Science (DNYS 12 months), Diploma in Ayurveda Practices (DAP 12 months), Diploma in Hospital Management (DHM 12 months), Diploma in Health and Sanitation (DHS 12 months), Diploma in Industrial Hygiene (DIH 12 months)
Diploma Pharmaceutical Quality Assurance and Quality Control (DPQAQC)
The programme targets candidates with keen interest in the Quality Assurance and Quality Control divisions of an industry. The Diploma programme is aimed to make the participant learn the:
- Need of QA & QC
- Where and when to apply
- Current norms and regulations (for both compliance and non-compliance)
- Testing tools and techniques
- Overcoming QA problems and fixing methods
All the above as well as practical knowledge about the subject has been included as case studies, practice modules, online sessions and lectures, online classrooms and discussions etc. The sole aim remains to make ready candidates with ample and appropriate knowledge about varied quality issues, concerns, industry needs, techniques, legislative norms and precise knowledge for identifying and overcoming quality related problems arising at work place. With this knowledge the participant can confidently aim to apply for and be a part of healthcare production units, quality check divisions of manufacturing plants and even quality auditing boards and regulatory authorities.
Programme Modules
International Affiliation with
Programme Modules
International Affiliation with
Module 1: Quality Assurance and Quality Control in pharmaceutical industry
- Basics of Quality
- GMP Regulations: USFDA, EU, TGA, ICH, WHO
- Need of Quality
- Introduction to QA and QC
Module 2: Qualification and Validation
- Introduction to Validation
- Validation Master Plan
- Qualification: DQ, IQ, OQ, PQ, Calibration and Maintenance
- Validation: Process Validation, Analytical Method Validation, Water System Validation, HVAC, Cleaning Validation, Sterilisation Validation, Computer System Validation
Module 3: Quality Assurance and Quality Control- Possible Problems and Fixes
- Deviations
- Out of Specification (OOS) and Out of Control (OOT)
- Change Control
- Compliance
- CAPA
Module 4: Quality Statistical Tools and Techniques
- Quality Testing Tools
- Quality Assurance Tools
- Statistical Process Control
- Quality Management Tools
Module 5: Quality Testing and its types
- Analytical Techniques
- API testing
- Raw material testing; Excipients, intermediates and container closure
- Finished Product Testing
- Stability Testing
- Microbiological testing
Module 6: Drug Stability Study
- ICH Stability Testing
- Stability Testing of new Drug Substances and Products
- ICH Impurities Testing
Module 7: Regulatory Authorities and Quality Certification
- Global Regulatory Authorities; USFDA, EMA, UKMHRA, TGA, CDSCO
- ICH, WHO, PIC/S
Module 8: ICH Guidelines and ISO
- International Council on Harmonisation (ICH)
- ICH Guidance on: Quality, Safety, Efficacy and Multidisciplinary
- International Organization for Standardization (ISO)
- ISO 9000, 9001:2015 series
Module 9: Quality Risk Management
- ICH Quality Risk Management
- Guidelines on Quality Risk Management: USFDA, WHO, EU
Module 10: Total Quality Management
- ICH Pharmaceutical Quality System
- Quality Culture
- Quality Policy
- Quality Commitment
- Continuous Improvement: Kaizen, LEAN Six Sigma
Module 11: Addressing Internal and External Quality Issues
- Quality Defects
- Defect Identification Tools
- Root Cause Investigation
- Deviation, Change Control, CAPA. Complaint and Recall Handling
Module 12: Inspections and Audits Types, Non Compliance handling
- Types of Audits and Inspections
- Regulatory Audits (FDA, MHRA, PMDA, TGA, DCGI)
- Audit Observations, Response letters, Warning letters, Revocation of GMP license
- Breach Reports
Module 13: Good Documentation Practices, SOPs, Protocols, etc
- Good Documentation Practices (GDP)
- Data Integrity
- SOP, Analytical Test Protocol, Work Instruction, Checklists, Log books
Module 14: Online Laboratory demonstration of analytical techniques
- Qualitative and Quantitative techniques
- Analytical and Bioanalytical methods
- Titrimetric, Electrochemical, Spectroscopic and Chromatographic techniques
Module 15: Software Based Analysis
- Empower
- Topspin
- Lab solution
- LIMS
Module 16: Industry Based Case Studies
Eligibility
10 + 2.
Programme Duration
The duration to complete this programme is 1 year.
Registration
The registration dates for this programme run by the Institute are updated timely on the webpage. Effective online learning tools incorporated into the design of the webpage make the programme lectures, online live classes and study material easily accessible. This gives a huge window of self-regulated and self-paced performance to the participants.
Programme Deliverables
A comprehensive study material for all the modules in hard copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
- Assignments for all the programme modules for continuous evaluation and guidance.
- Interactive or online live sessions on all key areas of the programme giving all flexibility to the participants.
- Online classes for all the modules will be conducted on the weekends. Moreover, a doubt clearing session will also be scheduled before the examination
- All the efforts are made by IGMPI faculty members to make the entire programme modules easily understandable.
- Assessment and evaluation for all the programme modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
- At the end of each programme modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
- All learning and training delivery initiatives shall be conducted in English.
Examination and Certification
All the participants are expected to appear for an online exam and are also obliged to submit assignments after each module. After successful completion, the participants will be awarded Diploma in Pharmaceutical Quality Assurance and Quality Control by Faculty of Good Manufacturing Practice, IGMPI. For all the above-mentioned modules, Online Classes (Online mode) or face-to-face classes (Regular mode), elaborate programme material, self-assessment assignments would be provided by the Institute. Details get updated on the webpage as well.
Placement Assistance & Corporate Relations
The Institute has partnered with many organizations for providing with placement assistance to in its participants. Besides, it has a robust placement cell comprised of senior level Human Resources professionals and Talent Acquisition experts which maintains close links with business and industry. This cell is continuously engaged in promoting the employability of our participants and encouraging the concerned Human Resources department and Hiring Managers to recruit/hire our participants for their vacant positions. The efforts of our placement cell also include helping with professional resume writing & interview skills.
In recent months the Institute has witnessed more and more participation from professionals working with global pharmaceutical, healthcare like Johnson & Johnson, Roche, Novartis, Merck & Co., AbbVie, GlaxoSmithKline, Pfizer, Sanofi, Bayer, Eli Lilly & Co., Novo Nordisk, Teva Pharmaceutical Industries, Dr. Reddy’s Laboratories, Lupin Limited, Torrent Pharmaceutical Ltd., Cipla Limited, Emcure Pharmaceuticals, Sun Pharmaceutical Limited, Mankind Pharma, Ajanta Pharma, IPCA Laboratories etc. The IGMPI’s Corporate ResourceDivision actively recommends our students and training participants for various jobrequirements and specialized roles to Human Resource, Talent Acquisition as well as the headsof various departments in Pharmaceutical, Healthcare industries on regular basis.
Future career prospects
QA & QC are quite essential parts of pharmaceutical, biotech and all other verticals of the healthcare industry. the two find application in other industry types as well but since healthcare is the most dynamic industry and the one concerned with health and well-being of the population, QA & QC are highly treated and most regarded divisions to settle in. thus the career prospects and opportunities are never ending.
With this important role in the industry, sense of responsibility is expected from the joining employee and of programme a thorough and complete knowledge of the work area. Our online programme works to prepare candidates for the same. The interactive online programmes thus has been tailor made to suit needs of employed as well as yet to be employed candidates targeting to take over the quality assurance and quality control divisions of the industry.
Diploma in Pharmaceutical Management (DPM)
As the name entails marketing tools and tactics are at the core of the curriculum. History of pharmaceutical sales and marketing, the revolutionary incidences of the industry, the rules, the framework (legal and ethical both) all are touched in details and the candidates are instructed about each possible theoretical as well as practical aspect of this field of work.
pharmaceutical sales and marketing and much needed aspect of any product’s life cycle. The success or failure of a product, a brand and a company emphasises the role and effort of the marketing team behind it. So for all it becomes necessary to have well learned and trained professionals into this job which can frame or even de-face the reputation of a company. Thus, the PM programme by IGMPI has been designed keeping in view the critical nature of this kind of work and role of professionals in this field.
The pharma marketing team has to have specific characters like:
- Ready to take ownership of the product and put effort into its marketing whole heartedly
- Have appropriate knowledge of the marketing tools to enable positive marketing of the products
- To be able to handle large teams and interact with technical teams involved in development of the product
- Have a sharp mind to evaluate prospects for a product and be vigilant of competitor products
- Have knowledge of the changing face of the industry, new odes of marketing and better methods of reaching the customer
Programme Modules
International Affiliation with
Programme Modules
International Affiliation with
Module 1: Introduction to Pharmaceutical Industry and Pharma Product Management
Module 2: Principles of Management
Module 3: Pharmaceutical Marketing Management, Pharmaceutical segmentation outlook
Module 4: Pharmaceutical Sales & Advertising Management
Module 5: Guidelines of Visual Aids and Input Designing
Module 6: Pharmaceutical Logistics Management
Module 7: Fundamentals of Drug Discovery
Module 8: Healthcare System in US, India and Other Countries
Module 9: Pharmaceutical Industry: Models and Market Research
Module 10: Pharmacoeconomics (Pricing Laws)
Module 11: Legal and Regulatory Framework of Pharmaceutical Industry
Module 12: Promotion in Pharmaceutical industry: Communication Model, Effective promotional communication
Module 13: Business Communication in the Pharmaceutical Industry
Module 14: Digital Marketing, Relationship marketing –KOL Management
Module 15: E-Commerce Management
Module 16: Entrepreneurship Development in Pharmaceutical Industry
Module 17: Industry Based Case Studies
Eligibility
10 + 2.
Programme Duration
The duration to complete this programme is 1 year.
Registration
The registration dates for this programme run by the Institute are updated timely on the webpage. Effective online learning tools incorporated into the design of the webpage make the programme lectures and study material easily accessible. This gives huge window of self-regulated and self-paced performance to the participants.
Programme Deliverables
A comprehensive study material for all the modules in hard copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
- Assignments for all the programme modules for continuous evaluation and guidance.
- Interactive or online live sessions on all key areas of the programme giving all flexibility to the participants.
- Online classes for all the modules will be conducted on the weekends. Moreover, a doubt clearing session will also be scheduled before the examination
- All the efforts are made by IGMPI faculty members to make the entire programme modules easily understandable.
- Assessment and evaluation for all the programme modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
- At the end of each programme modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
- All learning and training delivery initiatives shall be conducted in English.
Examination and Certification
All the participants are obliged to timely submit completed assessment assignments (during the programme, usually after every module) and appear for an online exam at the end of the programme. After successful completion, the participants will be awarded Diploma in Pharmaceutical Management by Faculty of Product Management, IGMPI. For all the above-mentioned modules, Online Classes (Online mode) or face-to-face classes (Regular mode), elaborate programme material, self-assessment assignments would be provided by the Institute. Details get updated on the webpage as well.
Placement Assistance & Corporate Relations
The Institute has partnered with many organizations for providing with placement assistance to its participants. Besides, it has a robust placement cell comprised of senior level Human Resources professionals and Talent Acquisition experts which maintains close links with business and industry. This cell is continuously engaged in promoting the employability of our participants and encouraging the concerned Human Resources department and Hiring Managers to recruit/hire our participants for their vacant positions. The efforts of our placement cell also include helping with professional resume writing & interview skills.
In recent months the Institute has witnessed more and more participation from professionals working with global healthcare like Dr. Reddy’s Laboratories, Cipla, Cadila Healthcare, Gland Pharma, Aurobindo Pharmaceuticals, Lupin, Torrent Pharmaceuticals, Zydus Lifesciences, Accenture, Tata Consultancy Services, Bayer, Galentic Pharma (India) Pvt. Ltd., Wipro, Genpact, Emcure, Lupin, Mankind, Johnson & Johnson, Jubilant Lifesciences, Roche, Zydus, Sun Pharmaceuticals, Lupin, Eli Lilly & Company, Pfizer, Merck, Astrazeneca, Glenmark, Alembic, Hetero, Thermo, Lakme, Nivea, Oriflame, Loreal, Lotus, etc. The IGMPI’s Corporate Resource Division actively recommends our students and training participants for various job requirements and specialized roles to Human Resource, Talent Acquisition as well as the heads of various departments in Pharmaceutical, Healthcare industries on regular basis.
Future career prospects
Pharmaceutical sales and marketing is a huge industry with tremendous scope for science as well as non-science graduates. The industries call in for talented and professional individuals who are ready to take challenging career path in this industry. Devotion and will to excel works best for team leaders with excellent knowledge of marketing skills and tools and potential to work dedicate time and effort to succeed in the industry.
IGMPI offers professional and industry oriented training in Pharmaceutical Marketing thus opening doors for entry into the industry. Diploma in PSMM adds advantage for those already in the industry as a tag of professional training and those new to the industry feel comfortable with backing of apt and up-to-date information and training required to have sustained growth in the industry.
Diploma in Pharmaceutical Management is a comprehensive programme offering candidate with knowledge and practically important information about the industry type, working modalities, methodology used, techniques of quality control, drug development precisely but whole emphasis remains on Pharmaceutical Management.
Diploma in Pharmaceutical Packaging (DPP)
Packaging is pervasive and essential and plays a significant role in the quality of Pharmaceutical products by providing protection from environmental, chemical, and physical challenges.This protection can be as simple as preventing breakage of the product to providing barriers to moisture, oxygen and other environmental conditions.
Pharmaceutical packaging(or drug packaging) is the packages and the packaging processes for pharmaceutical preparations. It involves all of the operations from production through distribution channels to the end consumer.Pharmaceutical Packaging is a very specialized area. Packaging of pharmaceutical products requires specific knowledge in areas such as packaging material, packaging machinery, legislation, risk and safety. This programme will provide professionals with a good basic grounding of what is required for the packaging of pharmaceutical and health care products.
Programme Modules
International Affiliation with
Programme Modules
International Affiliation with
Module 1: Introduction to Pharma Product Management
Module 2: Pharmaceutical Logistics management
Module 3: Introduction to Packaging Science and Technology
Module 4: Packaging Trends: A Global Scenario
Module 5: Variation in Packaging
Module 6: Pharmaceutical Packaging Material
Module 7: Packaging Closures and Sealing Systems
Module 8: Logistics Packaging for Pharmaceutical Marketing
Module 9: Total Quality Management and GMP: Quality Risk Management, Assessing Quality Concerns at Different Work Units or Areas
Module 10: Testing and Quality Control
Module 11: Shelf life evaluation of Pharmaceutical Packaging
Module 12: Laws for Pharmaceutical Packaging and related issues
Module 13: Industry based Case studies
Eligibility
10 + 2.
Programme Duration
The duration to complete this programme is 1 year.
Registration
The registration dates for this programme run by the Institute are updated timely on the webpage. Effective online learning tools incorporated into the design of the webpage make the programme lectures and study material easily accessible. This gives huge window of self-regulated and self-paced performance to the participants.
Programme Deliverables
A comprehensive study material for all the modules in hard copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
- Assignments for all the programme modules for continuous evaluation and guidance.
- Interactive or online live sessions on all key areas of the programme giving all flexibility to the participants.
- Online classes for all the modules will be conducted on the weekends. Moreover, a doubt clearing session will also be scheduled before the examination
- All the efforts are made by IGMPI faculty members to make the entire programme modules easily understandable.
- Assessment and evaluation for all the programme modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
- At the end of each programme modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
- All learning and training delivery initiatives shall be conducted in English.
Examination and Certification
All the participants are obliged to timely submit completed assessment assignments (during the programme, usually after every module) and appear for an online exam at the end of the programme. After successful completion, the participants will receive a certificate of Diploma in Pharmaceutical Packaging, IGMPI. For all the above-mentioned modules, Online Classes (Online mode) or face-to-face classes (Regular Mode), elaborate programme material, self-assessment assignments would be provided by the Institute. Details get updated on the webpage as well.
Placement Assistance & Corporate Relations
The Institute has partnered with many organizations for providing with placement assistance to its participants. Besides, it has a robust placement cell comprised of senior level Human Resources professionals and Talent Acquisition experts which maintains close links with business and industry. This cell is continuously engaged in promoting the employability of our participants and encouraging the concerned Human Resources department and Hiring Managers to recruit/hire our participants for their vacant positions. The efforts of our placement cell also include helping with professional resume writing & interview skills.
In recent months the Institute has witnessed more and more participation from professionals working with global healthcare like Uflex Ltd., Anuroop Packaging Ltd., IPCA, Zydus, Unichem, Sun Pharmaceuticals, Hetero, Torrent Pharmaceutical, Cipla, Lupin, Johnson & Johnson, Pfizer, Roche, Intas, Asrezenenca, Gland, Glenmark, Macleods, Jubilant Lifesciences, Novartis, Merck, Piramal, Dr. Reddy’s Laboratories, Hamdard, Lotus, Lakme, Khadi, Himalayan, etc. The IGMPI’s Corporate Resource Division actively recommends our students and training participants for various job requirements and specialized roles to Human Resource, Talent Acquisition as well as the heads of various departments in Pharmaceutical, Healthcare industries on regular basis.
Future career prospects
In today's world of global markets and stiff competition in every product along with increasing consumer demand, it becomes imperative for companies to explore ways to improve their productivity in terms of maintaining safety, using sustainable packaging materials, implementing flexible and standardised technology, and adopting proven management principles.Packaging Industry offers multi-disciplinary careers and requires specialised knowledge of the materials, processing, design, quality, and environmental trends.This programme is the perfect choice for professionals who want to get up-to-date easily on the most important aspects of pharmaceutical packaging.After completion of this programme, professionals can work as packaging engineer, pharmaceutical packaging consultant, packaging technician, packaging technologist, packaging analyst.
Diploma in Cosmetic Technology (DCT)
Cosmetic Technology is a Science of making cosmetics.The cosmetic field quickly applies cutting edge research to high value commercial products that has large impact on our lives and on the world’s economy. Cosmetic Technologies has been at the forefront of innovative personal care manufacturing for over twenty years.The programme gives extensive knowledge of Cosmetic Formulation, Manufacturing, Analysis and Marketing. It also covers the fundamental aspects cosmetic science that are necessary to understand material development, formulation, and the dermatological effect that result from the use of tissue products.
Programme Modules
International Affiliation with
Programme Modules
International Affiliation with
Module 1: Introduction to Cosmetics and Cosmoceuticals
- History and Evolution of Cosmetics Science
- Need for study of Cosmeceuticals
- New Advances in the field of Cosmeceuticals
Module 2: Anatomy and Physiology of Skin and Hair
- Anatomy and Physiology of Skin
- Pathology of skin hair and nails
- Process of skin healing
Module 3: Analytical Techniques for Cosmetic Products Evaluation
- Quantitative and Qualitative Methods of determination of Compounds
- Ion Exchange Chromatography, Size Exclusion Chromatography
- Analytical Techniques and Principles Gel Electrophoresis, HPLC
- Spectroscopical Methods of Analysis
Module 4: Product Development and Cosmetic Design and Formulations
- Process of Cosmetic Product Development
- Product Innovation and Development
- Formulation and Development
Module 5: Synthetic and Herbal Formulation Building Blocks
- Classification of Skin Cosmetics
- Additives used in the Skin Care Formulations
- Drug Excipient Interactions
- Formulation Requirements of Cosmetic Products
Module 6: Advanced Skin care Cosmetic Formulations
- Formulation of Cold Cream, Moisturizing Lotions, lotions, face serums, gels and sunscreens
- Evaluation of skin creams and lotions, face serums, gels and sunscreens
Module 7: Oral Care Cosmetic Formulations
- Formulation of Oral care products, Toothpastes, Tooth gels, Mouthwashes
- Evaluation of oral care formulations
Module 8: Colour Cosmetics and their Formulation
- Formulations of colour cosmetic products-Lipsticks, Nail lacquers, Rouge, Eye shadows
- Formulation of Tints (Lip Tints and Cheek Tints)
- Natural coloring Agents and Dyes
Module 9: Herbal Cosmeceuticals and its formulation
- Herbal and Natural Ingredients used in the formulations
- Herbal Skin care Cosmetic Products
- Herbal hair care formulations
Module 10: Various Ailment based formulations (Acne, Anti-aging, Allergy and Pigmentation etc.)
- Anti-acne, Anti-wrinkle Formulations
- Formulations to cure skin pigmentation, scars and marks
Module 11: Packaging techniques for cosmetic products
- Primary and Secondary Packaging Systems of Cosmetic Products
- Sustainable Packaging System of Cosmetic Products
- Global Cosmetic Packaging Scenario
Module 12: Good Manufacturing Practices & Quality Assurance in Cosmetic Technology
- GMP guidelines for Cosmetic Products
- Quality Audits Procedures
- Regulatory Compliances
Module 13: Cosmeceutical Marketing and Brand Management
- Introduction to Goods and Products
- FMCG-Fast Moving Consumer Goods
- Pharmaceutical market vs. FMCG Market
Module 14: Global Regulatory and IPR guidelines for Cosmetics
- Cosmetic Regulations of FDA
- Cosmetic Regulations of EU, US and Japan
- Global Regulatory Compliance and Regulations
Module 15: Industry based Case Studies
Eligibility
10 + 2.
Programme Duration
The duration to complete this programme is 1 year.
Registration
The registration dates for this programme run by the Institute are updated timely on the webpage. Effective online learning tools incorporated into the design of the webpage make the programme lectures and study material easily accessible. This gives huge window of self-regulated and self-paced performance to the participants.
Programme Deliverables
A comprehensive study material for all the modules in hard copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
- Assignments for all the programme modules for continuous evaluation and guidance.
- Interactive or online live sessions on all key areas of the programme giving all flexibility to the participants.
- Online classes for all the modules will be conducted on the weekends. Moreover, a doubt clearing session will also be scheduled before the examination
- All the efforts are made by IGMPI faculty members to make the entire programme modules easily understandable.
- Assessment and evaluation for all the programme modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
- At the end of each programme modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
- All learning and training delivery initiatives shall be conducted in English.
Examination and Certification
All the participants are obliged to timely submit completed assessment assignments (during the programme, usually after every module) and appear for an online exam at the end of the programme. After successful completion, the participants will receive a certificate of Diploma in Cosmetic Technology by Faculty of Cosmetic Technology, IGMPI. For all the above-mentioned modules, Online Classes (Online mode) or face-to-face classes (Regular mode), elaborate programme material, self-assessment assignments would be provided by the Institute. Details get updated on the webpage as well.
Placement Assistance & Corporate Relations
The Institute has partnered with many organizations for providing with placement assistance to its participants. Besides, it has a robust placement cell comprised of senior level Human Resources professionals and Talent Acquisition experts which maintains close links with business and industry. This cell is continuously engaged in promoting the employability of our participants and encouraging the concerned Human Resources department and Hiring Managers to recruit/hire our participants for their vacant positions. The efforts of our placement cell also include helping with professional resume writing & interview skills.
In recent months the Institute has witnessed more and more participation from professionals working with global pharmaceutical like L'oréal, Clinique, Garnier, Johnson & Johnson, Cipla Ltd, Sun Pharmaceutical Ltd., The Himalaya Drug Company, Bioblush India, Procter & Gamble, Renee Cosmetics, Zydus Cadila Healthcare Ltd., Uriflame, Intas Pharmaceuticals Ltd., etc. The IGMPI’s Corporate Resource Division actively recommends our students and training participants for various job requirements and specialized roles to Human Resource, Talent Acquisition as well as the heads of various departments in Pharmaceutical, Healthcare industries on regular basis.
Future career prospects
There is a wide scope in the profession of cosmetology in India or in other countries. Cosmetology is the promising career for youth because of advancement in the life style. The personal care industry makes up 22 percent of India's market for consumer package goods and experts agree that India is full of opportunities and is a potential gold mine for many beauty and personal care companies.As a cosmetic professional you would be involved in the research and development, manufacturing and production of cosmetics, hair care, perfume and toiletry products.
Diploma in Herbal Cosmetics (DHC)
Herbal cosmetics are the recent trend in the field of beauty and fashion. Indian herbs and its significance are popular worldwide. Herbal cosmetic has growing demand in the world market and are a precious gift of nature.
Men and women prefer to use natural products for their personal care as these products supply the body with nutrients and provide satisfaction as these are free from synthetic chemical and have relatively less side-effects compared to the synthetic cosmetics.
Many Health Organizations currently recommend and encourages traditional herbal cures in natural health care programs as these drugs are easily available at low cost and are comparatively safe. Plants are used not only for the cosmetics but also for the dermatological disorder.
As compared to synthetic products, herbal cosmetics are safe to use. They are less allergenic, non toxic, tested and proven by dermatologists to be safe to use since they are made of natural ingredients.
Herbal cosmetics are the preparations containing phytochemicals from a variety of botanical sources which influence the function of skin and provide nutrients necessary for the healthy skin or hair.
The natural herbs and their products when used for their aromatic value in cosmetics preparations are termed as herbal cosmetics. Herbal products contain desirable physiological activity such as healing, smoothing appearance, enhancing and conditioning properties because of herbal ingredients. The knowledge of medicinal plants used by the people seems to be well known to its culture and tradition. Some of the plants were found to have dual use, both curative and cosmetic.
The growing demand has created new avenues in cosmeceutical market as there is a wide selection to choose from natural cosmetics.
Programme Modules
International Affiliation with
Programme Modules
International Affiliation with
Module 1: Introduction to Cosmetics and Cosmeceuticals
- History and Evolution of Cosmetics Science
- Need for study of Cosmeceuticals
- New Advances in the field of Cosmeceuticals
Module 2: Anatomy and Physiology of Skin and Hair
- Anatomy and Physiology of Skin
- Pathology of skin hair and nails
- Process of skin healing
Module 3: Analytical Techniques for Cosmetic Products Evaluation
- Quantitative and Qualitative Methods of determination of Compounds
- Ion Exchange Chromatography, Size Exclusion Chromatography
- Analytical Techniques and Principles Gel Electrophoresis, HPLC
- Spectroscopical Methods of Analysis
Module 4: Herbal Drug Technology
- Definition of herb, herbal medicine, herbal medicinal product
- Herbal drug preparation Source of Herbs, Selection, identification and authentication of herbal materials
- Processing of herbal raw material
Module 5: Good Manufacturing Practices & Quality Assurance in Cosmetic Technology
- GMP guidelines for Cosmetic Products
- Quality Audits Procedures
- Regulatory Compliances
Module 6: Herbal Product Development and Cosmetic Formulations
- Methods of Herbal Product Development
- Screening, Standardization and Identification Procedures
- Process of Cosmetic Product Development
Module 7: Herbal Hair and Oral Care Cosmetics
- Formulation of Herbal Hair shampoos, Herbal Hair Conditioners, Hair Oils
- Formulations of Herbal Tooth Pastes, Mouth Washes, Oral Floss
- Evaluation of oral care formulations
Module 8: Herbal Skin care Cosmetics
- Formulation of Herbal skin care products-Herbal creams, herbal face washes
- Formulation of Herbal moisturizers and serums
Module 9: Production and Marketing Management in Herbal Cosmetics
- Introduction to Goods and Products
- FMCG-Fast Moving Consumer Goods
- Pharmaceutical market vs. FMCG Market
Module 10:Industry based Case Studies
Eligibility
10 + 2.
Programme Duration
The duration to complete this programme is 1 year.
Registration
The registration dates for this programme run by the Institute are updated timely on the webpage. Effective online learning tools incorporated into the design of the webpage make the programme lectures, online live classes and study material easily accessible. This gives a huge window of self-regulated and self-paced performance to the participants.
Programme Deliverables
A comprehensive study material for all the modules in hard copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
- Assignments for all the programme modules for continuous evaluation and guidance.
- Interactive or online live sessions on all key areas of the programme giving all flexibility to the participants.
- Online classes for all the modules will be conducted on the weekends. Moreover, a doubt clearing session will also be scheduled before the examination
- All the efforts are made by IGMPI faculty members to make the entire programme modules easily understandable.
- Assessment and evaluation for all the programme modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
- At the end of each programme modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
- All learning and training delivery initiatives shall be conducted in English.
Examination and Certification
All the participants are obliged to timely submit completed assessment assignments (during the programme, usually after every module) and appear for an online exam at the end of the programme. After successful completion, the participants will be awarded Diploma in Herbal Cosmetics by IGMPI. For all the above mentioned modules elaborate programme material, self-assessment assignments and project work details would be provided by the Institute from time to time. Details get updated on the webpage as well.
Placement Assistance & Corporate Relations
The Institute has partnered with many organizations for providing with placement assistance to its participants. Besides, it has a robust placement cell comprised of senior level Human Resources professionals and Talent Acquisition experts which maintains close links with business and industry. This cell is continuously engaged in promoting the employability of our participants and encouraging the concerned Human Resources department and Hiring Managers to recruit/hire our participants for their vacant positions. The efforts of our placement cell also include helping with professional resume writing & interview skills.
In recent months the Institute has witnessed more and more participation from professionals working with global healthcare like Himalaya Herbals, Lotus Herbals, Khadi Natural, Vaadi Herbals, Just Herbs, Biotique, Forest Essentials, Ayur Herbals, VLCC, JOVEES Herbal, Hamdard Laboratories, Baidyanath Pharmaceuticals, Dabur India Limited, Patanjali, Soulflower, Fabiindia, Organic India, Medimix, Mamaearth, Uflex Ltd., Anuroop Packaging Ltd., IPCA, Zydus, Unichem, Sun Pharmaceuticals, Hetero, Torrent Pharmaceutical, Cipla, Lupin, Johnson & Johnson, Pfizer, Roche, Intas, Asrezenenca, Gland, Glenmark, Macleods, Jubilant Lifesciences, Novartis, Merck, Piramal, Dr. Reddy’s Laboratories, Lotus, Lakme, Khadi, Himalayan, etc. The IGMPI’s Corporate Resource Division actively recommends our students and training participants for various job requirements and specialized roles to Human Resource, Talent Acquisition as well as the heads of various departments in Pharmaceutical, Healthcare industries on regular basis.
Future career prospects
There is a wide scope in the profession of herbal cosmetology in India or in other countries. Herbal Cosmetology is the promising career for youth because of the world wide growing market of the herbal product due to its safe and effective nature. The personal care industry makes up vast of India's market for consumer package goods and experts agree that India is full of opportunities and is a potential gold mine for many beauty and personal care companies. As a cosmetic professional you would be involved in the research and development, manufacturing and production of cosmetics, hair care, perfume and toiletry products.
Diploma in Environment Health and Safety (DEHS)
Every organization works hard to gain success and to be outstanding in the market. However, in this world of tough competition, any breach on safety, health and environment can become a serious hurdle to this path of success. Moreover as it is generally said that a successful business is in which all those involved are satisfied. This directly implies to safety at work and a secure environment where all those working are satisfied and work at their best.
Our global economy is increasing at a very faster pace, and it has been well projected that India has a big role in it. Although, we can’t expect a real benchmark of success until we are truly qualified in all the aspects. As it is evident that this sector of Environment Health and Safety has been neglected and not to mention those numerous accidents that occur frequently which entangles an organization aftermath in its consequences.
Thus seeing the rapid growth in infrastructure and more and more investments for industrial establishments there is increase in demand for HSE experts and for those seeking a successful ubiquitous career in this field should take this programme specially designed with an international perspective.
Programme Modules
International Affiliation with
Programme Modules
International Affiliation with
Module 1: Introduction to Environment Health and Safety (EHS)
Module 2: Industry Hazard Identification & Preventive Technique
Module 3: An understanding of the ISO 14001(Environmental Management Systems) & ISO 45001(Occupational Health and Safety Management Systems)
Module 4: An Overview on Legal aspects in Environment, Health and Safety
Module 5: Environment Monitoring & Measurement
Module 6: Emergency planning
Module 7: Health screening measures
Module 8: Socio-economic aspects of occupational health and safety
Module 9: Introduction to Chemical Safety
Module 10: Industry based case studies
Eligibility
10 + 2.
Programme Duration
The duration to complete this programme is 1 year.
Registration
The registration dates for this programme run by the Institute are updated timely on the webpage. Effective online learning tools incorporated into the design of the webpage make the programme lectures, online live classes and study material easily accessible. This gives a huge window of self-regulated and self-paced performance to the participants.
Programme Deliverables
A comprehensive study material for all the modules in hard copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
- Assignments for all the programme modules for continuous evaluation and guidance.
- Interactive or online live sessions on all key areas of the programme giving all flexibility to the participants.
- Online classes for all the modules will be conducted on the weekends. Moreover, a doubt clearing session will also be scheduled before the examination
- All the efforts are made by IGMPI faculty members to make the entire programme modules easily understandable.
- Assessment and evaluation for all the programme modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
- At the end of each programme modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
- All learning and training delivery initiatives shall be conducted in English.
Examination and Certification
All the participants are obliged to timely submit completed assessment assignments (during the programme, usually after every module) and appear for an online exam at the end of the programme. After successful completion, the participants will be awarded Diploma in Environment Health and Safety by Faculty of Environment Health and Safety, IGMPI. For all the above mentioned modules elaborate programme material and self-assessment assignments would be provided by the Institute from time to time. Details get updated on the webpage as well.
Placement Assistance & Corporate Relations
The Institute has partnered with many organizations for providing with placement assistance to its participants. Besides, it has a robust placement cell comprised of senior level Human Resources professionals and Talent Acquisition experts which maintains close links with business and industry. This cell is continuously engaged in promoting the employability of our participants and encouraging the concerned Human Resources department and Hiring Managers to recruit/hire our participants for their vacant positions. The efforts of our placement cell also include helping with professional resume writing & interview skills.
In recent months the Institute has witnessed more and more participation from professionals working with global healthcare like Big 4, Accenture, SGS, Fortune 100, Uniqus Consultech Ltd., ESG Book, Vision 360, TCS, Ckinetics, CSE(Centre for Science and Environment, TERI (The Energy and Research Institute), Iforest, CEEW (Council on Energy, Environment and Water ), CPR (Centre for Policy and Research ), Centre for Energy and Environment Sustainability, WWF (Worl wildlife Fund ), IWMI (International Water Management Institute ), Greenpeace, 350.org, ATREE, Chintan Environmental Research and Action Group, Sankalp Taru Foundation, TBS, Tarun Bharat Sangh, Mukti, Aquatech Systems Asia Pvt. Ltd., Nalco Water India Ltd.(Ecolab),ABM Environment Consultants, Eco Paryavaran Engineers and Consultants Pvt. Ltd., Enviro, Paramount Ltd., Enviro Control Associates India Pvt Ltd., Energy Tech Solutions, CVR Labs, EnVision Environmental Services, Mindtree Ltd., Siemens, Wipro Limited, Astra Zeneca, Novartis Healthcare, Accenture, Larsen & Toubro Limited, Roche Pharmaceutical, PI Industries Ltd., Ericsson, Teva Pharmaceuticals, ICON Plc., Suven Life Sciences, Infosys Limited, Syneos Health Clinical, Eli Lilly and Company, etc. The IGMPI’s Corporate Resource Division actively recommends our students and training participants for various job requirements and specialized roles to Human Resource, Talent Acquisition as well as the heads of various departments in Pharmaceutical, Healthcare industries on regular basis.
Future career prospects
Diploma in Environment Health and Safety is a professional programme targeted to cater the occupational safety requirement of trained professionals. The information, guidance, practical training and off programme completion certificate will provide the participant with not one but many opportunities in the industry. This would come true in form of job roles and positions like that of HSE Executive, HSE Auditors, Head-HSE, HSE Managers, safety officers in any industry or relevant organization and many more.
Diploma in Medical Coding (DMC)
Medical coding is the first step of the medical billing process. Right coding leads to unambiguous billing process sufficing the needs of both service provider as well as the claimer. Healthcare system has a huge array of procedures, treatments, services and products made available to the patient’s. This makes accurate medical coding process even more important and therefore necessitates the need of skilled and proficient medical coders.
HCPCS, ICD and CPT are the essential medical coding textbooks or rule books. Since it is a centralized system, standardization of coding terminology is essential and thus, these textbooks are the reference point. Medical coding professional need to have complete and accurate knowledge of the medical coding norms and regulations alongside with apt dedication for their field! This is necessary as these people are trusted by the patients as well as the service providers (Doctors, clinicians, insurance companies etc.). They need to be dependable with all the crucial medical information of the patient plus is reliable for adequately coding the documented information which is used in the billing process.
Diploma Programme in medical coding is entitled to be the strongest tools for anybody targeting to enter into medical coding world. While being honest, organized, dependable and detail oriented are the personal traits which might help an individual to be a trustworthy medical coder. Knowledge of the medical terminology, good grip over the medical coding rule books, appropriate training about the industry and skills to use varied medical coding software is much needed to excel in this industry. CHMR offers this and much more in its Diploma programmes in Medical Coding.
The programme has been designed by industry experts and thus is full of examples and citation from the real world. The information offered throughout the programme has been diligently marked to be most recent and updated. This includes all the knowledge about the medical coding literature. The case studies have been appropriately interwoven into the programme modules so as to make learning more appropriate.
CHMR makes available this medical coding programme as a Regular and online interactive programme and thus the benefits it entails within are numerous. Like:
- Comprehensive information, clearly targeting the medical coding industry
- Well-structured programme, intelligently put together under guidance of industry experts from same field
Programme Modules
International Affiliation with
Programme Modules
International Affiliation with
Module 1:Introduction to Medical Coding
Module 2:Basics of Anatomy and Physiology
Module 3:Medical Coding and terminologies in association with Diseases
Module 4:Introduction to International Classification of Diseases (ICD)
Module 5:Certain Infectious and Parasitic Diseases (A00-B99)
Module 6:Neoplasms (C00-D49)
Module 7:Diseases of the Blood and Blood-forming Organs and Certain Disorders Involving the Immune Mechanism (D50-D89)
Module 8:Endocrine, Nutritional, and Metabolic Diseases (E00-E89)
Module 9:Mental, Behavioral and Neurodevelopmental Disorders (F01-F99)
Module 10:Diseases of Nervous System (G00-G99)
Module 11:Diseases of Eye and Adnexa (H00-H59)
Module 12:Diseases of Ear and Mastoid Process (H60-H95)
Module 13:Diseases of Circulatory System (I00-I99)
Module 14:Diseases of Respiratory System (J00-J99)
Module 15:Diseases of Digestive System (K00-K94)
Module 16:Diseases of Skin and Subcutaneous Tissue (L00-L99)
Module 17:Diseases of the Musculoskeletal System and Connective Tissue (M00-M99)
Module 18:Diseases of Genitourinary System (N00-N99)
Module 19:Pregnancy, Childbirth, and the Puerperium (O00-O9A)
Module 20:Newborn (Perinatal) Guidelines (P00-P96)
Module 21:Congenital Malformations, Deformations, and Chromosomal Abnormalities (Q00-Q99)
Module 22:Symptoms, Signs, and Abnormal Clinical and Laboratory Findings, Not Elsewhere Classified (R00-R99)
Module 23:Injury, Poisoning, and Certain Other Consequences of External Causes (S00-T88)
Module 24:External Causes of Morbidity (V00-Y99)
Module 25:Factors Influencing Health Status and Contact with Health Services (Z00-Z99)
Module 26:Healthcare Common Procedure Coding System (HCPCS)
Module 27:Current Procedural Terminology (CPT)
Module 28:Modifiers
Module 29:Evaluation and Management Guidelines for Medical coding professionals
Module 30:Radiology and Anaesthesia Guidelines for Medical coding professionals
Module 31:Surgery Guidelines for Medical coding professionals
Module 32:Pathology and Laboratory and Medicine Services and Procedures Guidelines for Medical coding professionals
Module 33:Health Insurance Portability and Accountability Act (HIPAA)
Module 34:Case Studies
Eligibility
10 + 2.
Programme Duration
The duration to complete this programme is 1 year.
Registration
The registration dates for this programme run by the Institute are updated timely on the webpage. Effective online learning tools incorporated into the design of the webpage make the programme lectures, online live classes and study material easily accessible. This gives a huge window of self-regulated and self-paced performance to the participants.
Programme Deliverables
A comprehensive study material for all the modules in hard copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
- Assignments for all the programme modules for continuous evaluation and guidance.
- Interactive or online live sessions on all key areas of the programme giving all flexibility to the participants.
- Online classes for all the modules will be conducted on the weekends. Moreover, a doubt clearing session will also be scheduled before the examination
- All the efforts are made by IGMPI faculty members to make the entire programme modules easily understandable.
- Assessment and evaluation for all the programme modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
- At the end of each programme modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
- All learning and training delivery initiatives shall be conducted in English.
Examination and Certification
All the participants are obliged to timely submit completed assessment assignments (during the programme, usually after every module) and appear for an online exam at the end of the programme. After successful completion, the participants will receive a certificate of Diploma in Medical Coding, IGMPI. For all the above-mentioned modules, Online Classes (Online mode) or face-to-face classes (Regular), elaborate programme material, self-assessment assignments would be provided by the Institute. Details get updated on the webpage as well.
Placement Assistance & Corporate Relations
The Institute has partnered with many organizations for providing with placement assistance to its participants. Besides, it has a robust placement cell comprised of senior level Human Resources professionals and Talent Acquisition experts which maintains close links with business and industry. This cell is continuously engaged in promoting the employability of our participants and encouraging the concerned Human Resources department and Hiring Managers to recruit/hire our participants for their vacant positions. The efforts of our placement cell also include helping with professional resume writing & interview skills.
In recent months the Institute has witnessed more and more participation from professionals working with global pharmaceutical and Healthcare like Visionary RCM Infotech, Episource Pvt. Ltd., GeBBSHealthcare Solutions, AGS Health, Vee Technologies, Cognizant Technology Solutions, Miramed Ajuba, CureMD Healthcare, AviaCode Incorporated, Change Healthcare, Omega Healthcare, Cotiviti India, Corro Health, Access Healthcare, Optum, etc. The IGMPI’s Corporate Resource Division actively recommends our students and training participants for various job requirements and specialized roles to Human Resource, Talent Acquisition as well as the heads of various departments in Pharmaceutical, Healthcare industries on regular basis.
Future career prospects
CHMR levels grounds and provides essential tools making the candidates confident before entering the field. The programme is structured in a way to provide knowledge, experience and training altogether. With basics set right and strong, the candidate is sure to excel in the medical coding industry.
Through the programme, aspirants gain good knowledge of medical coding industry leading to availability of best employment options. Medical coders use electronic data today to keep track of patient records easier, but there are still some medical offices that use paper filing. Medical Coders convert patient health information into alpha numeric codes. Law firms hire medical coders to investigate fraud claims. Also, Government agencies provide the opportunity to medical coders to work on projects that affect healthcare across the nation. Medical coders are seen in every type of Pharma and healthcare industry from clinics, to hospitals, insurance companies, diagnostic centres, and legal centres addressing health or life insurance issues and many more where they work as Drug Safety Experts, Maintenance manager, Quality analyst, Medical coder, Associate content analyst etc.
Diploma in Medical Records Technology (DMRT)
IGMPI offers a comprehensive short term programme in Medical Records Technology that provides important skills and knowledge to handle Medical Records in healthcare and hospital settings.
Medical Records Technology is a distinctive programme offering information pertaining to the operations of a healthcare facility. It is a science of managing the entire patient’s information related to collecting, filing and storing of important data. The programme involves study of patient charts, scanning Medical Records, medical coding of diseases, thereby ensuring compliance to regulations and retention schedules. The programme also emphasizes on destruction of Medical Records after their retention.
Medical Records Technology programme is specially designed to suit personnel providing medical records services and for personnel opting to take Medical Record Management as a career option in the field of life sciences and healthcare. This programme is intended to fill the shortage of skilled professionals in the field of Medical Record in hospital and industry settings.
The main objective of the programme is to upgrade technical skills and work efficiency in the core areas of health facility management through educating with the help of manual and electronic records. It also covers management of Inpatient and outpatient medical records and department registration services. As the programme involves modules on medical coding and ICD-10, this also covers generating statistics on morbidity and mortality by ICD-10 coding of diseases and diagnostic indexing. Additionally, the programme covers the legal issues related to Medical Records, audits by relevant agencies accuracy in filing, retrieving and keeping records.
Programme Modules
International Affiliation with
Programme Modules
International Affiliation with
Module 1:Medical Records- An Introduction
Module 2:Planning and Documentation: Medical Records
Module 3:Ethics and Legal Compliance
Module 4:Medical Coding
Module 5:International Classification of Diseases (ICD-10)
Module 6:Electronic Medical Records & it’s Challenges
Module 7:Architecture of Medical Records.
Module 8:Archival and Deletion of Medical Records
Module 9:Medical Claims and Processing
Module 10:Data Mining and Statistical Analysis
Module 11:Clinical Documentation Improvement
Module 12:Case Studies
Eligibility
10 + 2.
Programme Duration
The duration to complete this programme is 1 year.
Registration
The registration dates for this programme run by the Institute are updated timely on the webpage. Effective online learning tools incorporated into the design of the webpage make the programme lectures, online live classes and study material easily accessible. This gives a huge window of self-regulated and self-paced performance to the participants.
Programme Deliverables
A comprehensive study material for all the modules in hard copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
- Assignments for all the programme modules for continuous evaluation and guidance.
- Interactive or online live sessions on all key areas of the programme giving all flexibility to the participants.
- Online classes for all the modules will be conducted on the weekends. Moreover, a doubt clearing session will also be scheduled before the examination
- All the efforts are made by IGMPI faculty members to make the entire programme modules easily understandable.
- Assessment and evaluation for all the programme modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
- At the end of each programme modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
- All learning and training delivery initiatives shall be conducted in English.
Examination and Certification
All the participants are obliged to timely submit completed assessment assignments (during the programme, usually after every module) and appear for an online exam at the end of the programme. After successful completion, the participants will be awarded Diploma in Medical Records Technology by Centre for Health Management and Research (CHMR), IGMPI. For all the above-mentioned modules, Online Classes (Online mode) or face-to-face classes (Regular mode), elaborate programme material, self-assessment assignments would be provided by the Institute. Details get updated on the webpage as well.
Placement Assistance & Corporate Relations
The Institute has partnered with many organizations to provide placement assistance to in its participants. Besides, it has a robust placement cell comprised of senior-level human resources professionals and talent acquisition experts that maintains close links with business and industry. This cell is continuously engaged in promoting the employability of our participants and encouraging the concerned Human Resources department and hiring managers to recruit/hire our participants for their vacant positions. The efforts of our placement cell also include helping with professional resume writing & interview skills.
In recent months, the Institute has witnessed more and more participation from professionals working with global healthcare like Epic System Corporation, Meditech, AdvancedMD EHR, Binary Spectrum, Birlamedisoft Pvt. Ltd., NextGen Healthcare Information Systems, GE Healthcare, McKession, CureMD, Nextech, Kareo, Cerner Corporation, Epic Systems Corporation, Athenahealth, CareCloud Co., etc. The IGMPI’s Corporate Resource Division actively recommends our students and training participants for various job requirements and specialized roles to Human Resource, Talent Acquisition as well as the heads of various departments in Pharmaceutical, Healthcare industries on regular basis.
Future career prospects
India is projected to be ranked within the top three healthcare markets in terms of incremental growth by the year 2025. There are ample job opportunities in the field of medical science for the students of the medical records technician course. For those who want to work in the health-care medical records technology, the course can be a great choice for them. According to recent studies and statistics, a career in Medical Records Technology can be very rewarding. With the healthcare industry rising in significance, this programme can be very helpful in the current market scenario. This curriculum will assist you in becoming an efficient medical record manager. As a record manager, you will take care of maintaining patient’s medical records and perform statistical reporting and research. You'll learn how to use medical office software and billing strategies.
Medical Records Technicians are hired in both public and private hospitals and as well as in clinics, polyclinics, Diagnostic Centers, Medical Insurance Centers, Health Care Centers, Nursing Homes, Medical Colleges / Universities, Outpatient Care Centers etc. They are decently paid and they can anticipate good career growth. If you're looking to join a growing industry, you should consider a career in the field of medical record technology. The field is growing at a rapid pace. With more people spending more time in hospitals, more patients are using medical records for legal purposes. Health records are being scrutinized more and more by government agencies and insurance companies. As a result, this field is growing very fast and this will help you to find a job in the health care field that you'll love.
Diploma in Naturopathy and Yogic Science (DNYS)
There is an increasing demand for medicines from traditional systems in developing as well as developed countries because of their being into existence since a very long time thereby enjoying the reputation of being both safe and efficacious. Naturopathy is a field of health care that works with, and not against standard allopathic health care providers, such as medical doctors. The philosophy of Naturopathy is to work with the body, to assist it in its natural ability to heal and maintain health. Yoga is an ancient art, and a natural way of remaining fit and healthy. It also helps in improving mental health. Treatments are natural and non-invasive and include herbal remedies, lifestyle changes, and dietary modifications and in some case other complementary techniques including massage, acupuncture or aromatherapy.
Naturopathy Yoga practioners focus on the work closely with the patient’s own unique set of symptoms, reactions and Naturopathy & Yoga practioners focus on the work closely with the patient’s own unique set of symptoms, reactions and status. Natural health plays an important role for the holistic development of the personality. It is utmost important to have technical and scientific knowledge of all the components such as hydrotherapy, chromotherapy, diet therapy, magnet therapy etc. for promotion and preservation of health.
Programme Modules
International Affiliation with
Programme Modules
International Affiliation with
Module 1: Foundation of Natural Healing
- Principles and philosophy of naturopathy
- Historical development of naturopathic medicine
- Comparison with conventional medicine
Module 2: Herbal Medicine & Botanical Therapeutics
- Botanical Identification, Phytochemistry, Herbal Pharmacology
- Herbal Formulation, Medicinal Plant Families
- Therapeutic Uses: Application of specific herbs for various health conditions
- Safety and Toxicology: Understanding the safety profile of medicinal plants
- Herbal Medicine in Traditional Systems
Module 3: Nutritional Science for Holistic Health
- Nutritional principles and their role in health
- Therapeutic diets
- Nutritional supplements
Module 4: Human Anatomy & Physiology
- In-depth study of human anatomy and physiology
- Understanding the body's systems and functions
Module 5: Yogic Philosophy & Principles
- Exploration of ancient yogic principles and philosophy
- Integration of yogic principles into holistic health practices
- Understanding the mind-body connection in healing
Module 6: Asana Mastery: Postures for Wellness
- Practice and mastery of yoga postures (asanas)
- Alignment, breathing techniques, and modifications for individual needs
- Incorporation of asanas into holistic treatment plans
Module 7: Physical Medicine
- Therapeutic exercise
- Massage therapy
- Asana Mastery: Postures for Wellness
- Pranayama Techniques for Breath Control
Module 8: Pranayama Techniques for Breath Control
- Study and practice of pranayama (breath control) techniques
- Understanding the importance of breath in health and vitality
- Incorporation of pranayama into daily wellness routines
Module 9: Meditation Practices
- Mindfulness Meditation: Introduction to mindfulness principles
- Mindfulness meditation techniques
- Applications in stress reduction and mental health
- Techniques to enhance concentration and mental clarity
Module 10: Guide to Essential Drugs Prescribing Information
- Principles of Ayurveda, Unani, Siddha, and Homeopathy
- Herbal Formulations (Ayurveda, Unani, Siddha)
- Study of classical formulations and their indications
- Unani Pharmacology: Understanding the concept of Mizaj (temperament) and Akhlat (humors) in drug action
- Classification of drugs in Unani medicine
- Dosage and administration of Unani medicines
- Homeopathic Materia Medica
Eligibility
10 + 2.
Programme Duration
The duration to complete this programme is 1 year.
Registration
The registration dates for this programme run by the Institute are updated timely on the webpage. Effective online learning tools incorporated into the design of the webpage make the programme lectures, online live classes and study material easily accessible. This gives a huge window of self-regulated and self-paced performance to the participants.
Programme Deliverables
A comprehensive study material for all the modules in hard copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
- Assignments for all the programme modules for continuous evaluation and guidance.
- Interactive or online live sessions on all key areas of the programme giving all flexibility to the participants.
- Online classes for all the modules will be conducted on the weekends. Moreover, a doubt clearing session will also be scheduled before the examination
- All the efforts are made by IGMPI faculty members to make the entire programme modules easily understandable.
- Assessment and evaluation for all the programme modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
- At the end of each programme modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
- All learning and training delivery initiatives shall be conducted in English.
Examination and Certification
All the participants are expected to appear for an online exam and are also obliged to submit assignments after each module. After successful completion, the participants will be awarded Diploma in Naturopathy and Yoga Science by Centre for Health Management and Research, IGMPI. For all the above-mentioned modules elaborate programme material, self-assessment assignments and project work details would be provided by the Institute from time to time. Details get updated on the webpage as well.
Placement Assistance & Corporate Relations
The Institute has partnered with many organizations for providing with placement assistance to its participants. The robust placement cell comprises of senior level Human Resources professionals and Talent Acquisition experts which maintains close links with business and industry. We are engaged in promoting the employability of our participants by maintaining good rapport and relation with HR cell and recruiting managers of leading Food and Agriculture companies across the globe.The efforts of our placement cell also include helping with professional resume writing, interview skills & conducting mock interviews etc.
In recent Months the Institute has witnessed more and more participation from professionals working global healthcare and nutrition giants like Karma Ayurveda, Anjali Mukerjee Health Total, Patanjali Ayurved, Hindustan Unilever Ltd, Emami, Himalaya, NUTRIKALP, Sri Vaidya Kerala Ayurveda, AVA Sanjeevanam Ayurveda, Zandu care, Elastic Run, Wellintra Fitness, CosmoWellness, Ayuredic Soul Pvt. Ltd., Jiva Ayurveda, Sushruta Ayurvedic Therapy Center, Pure Natural Products Ltd., etc.
Future career prospects
The ultimate goal of the Naturopathy is to gradually and gently improve a patient’s health, enabling and motivating them to make lifestyle changes that they can maintain long term. Treatment is aimed at the illness causing symptoms, not at masking the individual symptoms themselves. The body is treated as a whole, the physical, emotional and mental aspects are all catered for. The naturopathy and yoga professional can opt different fields as per their choice, interest, expertise and knowledge. Some of the important broad disciplines where the professionals can consume themselves in Hospital and Healthcare Administration, Health Supervisor, Naturopathy Consultation as instructor, expert, specialist, Yoga aerobic Instructor, Yoga Instructor, Yoga Teacher, Therapists and Naturopaths, Trainer/ Instructor Health Club etc.
Diploma in Ayurveda Practices (DAP)
Ayurveda is the field of medication that is filling in conspicuousness for recent years. Ayurveda as an arising field spreads out a plenty of chances for youthful competitors who wish to seek after their vocation in the field of Ayurveda. A Diploma in Ayurvedic lays across a stage for student to learn top to bottom about the various medications and methods used to treat disease condition. The program is a coordinated investigation of Ayurveda as a field joining it with medical benefits.
Diploma in Ayurvedic course empowers student to be aware of the disease condition, substance properties of ayurvedic meds, the proportion, composition and amount in which it ought to be taken by patients and treatment therapy. They are imparted with the major standards of Ayurveda and its purposes in treating various illnesses. It fabricates serious areas of strength for a for the people who wish to seek after a higher capability in the field like Unhitched males and Bosses. As this course can be sought after by up-and-comers after class twelfth, the program goes about as an establishment for developing their insight about the subject.
Programme Modules
International Affiliation with
Programme Modules
International Affiliation with
Module 1: History of Ayurveda
Module 2: Sanskrit and Samhita
Module 3: Padartha Vigyana ( Ayurvedic Philosophy)
Module 4: Rachna Sharira (Anatomy)
Module 5: Kriya Sharira (Physiology)
Module 6: Rasa Shastra avam Bhaisajya Kalpana (Pharmaceuticals of Ayurveda)
Module 7: Dravyaguna (Material Medica of Ayurveda)
Module 8: Agadtantra, Vyavahra Ayurveda & Vidhi Vaidyaka (Toxicology & Jurisprudence )
Module 9: Nidan/ Vikriti Vigyana (Pathology)/Nadi Parkisa (Pulse Diagnosis)
Module 10: Svasthavritta Yoga (Personal & Social Hygiene including Dietetics )
Module 11: Charaka Samhita (A Classical text of Ayurveda )
Module 12: Kaya Chikitsa (General Medicine including Panchakarma, Rasayana & Vajikarana)
Module 13: Shalya Tantra (General Surgery & Para Surgical Techniques)
Module 14: Shalakya Tantra (ENT,Eye & Dentistry)
Module 15: Prastuti Tantra avam Stri Roga ( Gyanecology & Obstetrics )
Module 16: Kaumara Bhritya (Paediatrics)
Module 17: Medical Ethics
Module 18: Health Regulations
Module 19: Yoga
Eligibility
10 + 2.
Programme Duration
The duration to complete this programme is 1 year.
Registration
The registration dates for this programme run by the Institute are updated timely on the webpage. Effective online learning tools incorporated into the design of the webpage make the programme lectures, online live classes and study material easily accessible. This gives a huge window of self-regulated and self-paced performance to the participants.
Programme Deliverables
A comprehensive study material for all the modules in hard copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
- Assignments for all the programme modules for continuous evaluation and guidance.
- Interactive or online live sessions on all key areas of the programme giving all flexibility to the participants.
- Online classes for all the modules will be conducted on the weekends. Moreover, a doubt clearing session will also be scheduled before the examination
- All the efforts are made by IGMPI faculty members to make the entire programme modules easily understandable.
- Assessment and evaluation for all the programme modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
- At the end of each programme modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
- All learning and training delivery initiatives shall be conducted in English.
Examination and Certification
All the participants are expected to appear for an online exam and are also obliged to submit assignments after each module. After successful completion, the participants will be awarded Diploma in Ayurveda Practices (DAP) by Centre for Health Management and Research (CHMR), IGMPI. For all the above mentioned modules elaborate programme material, self-assessment assignments and project work details would be provided by the Institute from time to time. Details get updated on the webpage as well.
Placement Assistance & Corporate Relations
The Institute has partnered with many organizations for providing with placement assistance to its participants. The robust placement cell comprises of senior level Human Resources professionals and Talent Acquisition experts which maintains close links with business and industry. We are engaged in promoting the employability of our participants by maintaining good rapport and relation with HR cell and recruiting managers of leading Food and Agriculture companies across the globe. The efforts of our placement cell also include helping with professional resume writing, interview skills & conducting mock interviews etc.
In recent Months the Institute has witnessed more and more participation from professionals working global healthcare and nutrition giants like Karma Ayurveda, Anjali Mukerjee Health Total, Patanjali Ayurved, Hindustan Unilever Ltd, Emami, Himalaya, NUTRIKALP, Sri Vaidya Kerala Ayurveda, AVA Sanjeevanam Ayurveda, Zandu care, Elastic Run, Wellintra Fitness, CosmoWellness, Ayuredic Soul Pvt. Ltd., Jiva Ayurveda, Sushruta Ayurvedic Therapy Center, Pure Natural Products Ltd., etc.
Future career prospects
The ultimate goal of theAyurvedic Science is to gradually and gently improve a patients health, enabling and motivating them to make lifestyle changes that they can maintain long term. Treatment is aimed at the illness causing symptoms, not at masking the individual symptoms themselves. The body is treated as a whole, the physical, emotional and mental aspects are all catered for. The Ayurvedic Science professional can opt different fields as per their choice, interest, expertise and knowledge. Some of the important broad disciplines where the professionals can consume themselves in Hospital and Healthcare Administration, Health Supervisor, Naturopathy Consultation as instructor, expert, specialist, Yoga aerobic Instructor, Yoga Instructor, Yoga Teacher, Therapists and Nephropathy, Trainer/ Instructor Health Club, Area Sales Executive/Manager,Product Manager,Business Development Officeretc.
Diploma in Hospital Management (DHM)
Hospital Management and administration is concerned with the organization, coordination, planning, staffing, evaluating and controlling of health services for the masses. The primary objective is to provide quality healthcare to people and that too is a cost – effective manner. Professional hospital administrators have proven how institutions can be managed proficiently, economically and successfully in a given time period. Candidates with organizational skills and inclination for managerial activities are polished through training programmes about vast world of hospital management.Hospital management is all about managerial skills. Hospital Management professionals need to know and learn about varied levels and types of management starting from Managing operations, departments, supply chain, patient information to finance and staffing and more.
Programme Modules
International Affiliation with
Programme Modules
International Affiliation with
Module 1: Fundamentals of Healthcare Administration
Module 2: Organization and Management of Hospitals
Module 3: Epidemiology: Principles, Methods and limitations
Module 4: Research Methodology
Module 5: Managerial Economics
Module 6: Hospital Policies, Regulation and Medico-Legal Issues
Module 7: Hospital Record Management
Module 8: Financial & Accounting Management
Module 9: Human Resources Management
Module 10: Material and Waste Management
Module 11: Customer Management
Module 12: Disaster and Risk management
Module 13: Inventory Control & Material Mangement in Hospitals
Module 14: Operations Audits and Administrations
Module 15: Quality management & Patient Safety
Module 16: Entrepreneurship Development in Healthcare Industry
Module 17: Health Insurance & Managed Care
Module 18: Introduction to Healthcare Informatics and IT in Healthcare Services
Module 19: Artificial Intelligence in Healthcare Industry
Module 20: Case Studies
Eligibility
10 + 2.
Programme Duration
The duration to complete this programme is 1 year.
Registration
The registration dates for this programme run by the Institute are updated timely on the webpage. Effective online learning tools incorporated into the design of the webpage make the programme lectures, online live classes and study material easily accessible. This gives a huge window of self-regulated and self-paced performance to the participants.
Programme Deliverables
A comprehensive study material for all the modules in hard copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
- Assignments for all the programme modules for continuous evaluation and guidance.
- Interactive or online live sessions on all key areas of the programme giving all flexibility to the participants.
- Online classes for all the modules will be conducted on the weekends. Moreover, a doubt clearing session will also be scheduled before the examination
- All the efforts are made by IGMPI faculty members to make the entire programme modules easily understandable.
- Assessment and evaluation for all the programme modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
- At the end of each programme modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
- All learning and training delivery initiatives shall be conducted in English.
Examination and Certification
All the participants are expected to appear for an online exam and are also obliged to submit assignments after each module. After successful completion, the participants will be awarded Diploma in Hospital Management by Centre for Health Management and Research (CHMR), IGMPI. For all the above-mentioned modules, Online Classes (Online mode) or face-to-face classes (Regular mode), elaborate programme material, self-assessment assignments would be provided by the Institute. Details get updated on the webpage as well.
Placement Assistance & Corporate Relations
The Institute has partnered with many organizations for providing with placement assistance to in its participants. Besides, it has a robust placement cell comprised of senior level Human Resources professionals and Talent Acquisition experts which maintains close links with business and industry. This cell is continuously engaged in promoting the employability of our participants and encouraging the concerned Human Resources department and Hiring Managers to recruit/hire our participants for their vacant positions. The efforts of our placement cell also include helping with professional resume writing & interview skills.
In recent months the Institute has witnessed more and more participation from professionals working with global healthcare like Apollo Hospital, Fortis Healthcare, Healtharch India, Hospaccx Healthcare Consultancy, Max Hospital, Quality Konnect Consultant Pvt. Ltd., Medlinks India Mfg. Consulting, 3M Company, Cognizant Technology Solutions, Conduent, General Dynamics Corporation, McKesson Corporation, Nuance Communications, Altegra Health (Change Heathcare), ArborMetrix Inc., Citius Tech Inc., Dimensional Insight, United Health Group, Optum, GE Healthcare, Siemens Healthineers, Tata Consultancy Services, Hindustan Wellness, Navigant, Greystone Health, United Health Group, Optum, Philips, Abbott, GE Healthcare, Siemens Healthineers, Labcorp, etc. The IGMPI’s Corporate Resource Division actively recommends our students and training participants for various job requirements and specialized roles to Human Resource, Talent Acquisition as well as the heads of various departments in Pharmaceutical, Healthcare industries on regular basis.
Future career prospects
Hospital Management is concerned with the efficiency and cost effectiveness of all levels of health service. With the tremendous growth in the healthcare/hospital industry there are sufficient job opportunities for Hospital Managers in India and abroad.Most job opportunities in the field of Hospital Management are in hospitals, but one can also find openings in health agencies, laboratories and other health and allied services.Hospital Management positions varies from Head of the department to Chief executive officer at numerous organizational levels. Hospital Management programme adds advantage for those already in the industry as a tag of professional training and those new to the industry feel comfortable with backing of apt and up-to-date information and training required to have sustained growth in the industry.
Diploma in Health and Sanitation (DHS)
Sanitation is important for health and development around the world. Adequate sanitation, together with safe water, and good hygiene are foundation of good health and of social and economic development. Progress in one or more of these three components of good health can decrease the rates of morbidity and the severity of various diseases and increase the quality of life of extensive numbers of people, especially children, in developing countries.
All over the world, more than 800 children under the age of 5 years die every day from preventable diarrhoea-related diseases caused by lack of access to water, sanitation and hygiene. In addition, diarrhoea leads children to lose their appetites, resulting in malnutrition. Limited access to sanitation has become such a global issue that one out of every four childrensuffer from stunted growth. This leads to “irreversible physical and cognitive damage". The health sector has a powerful motivation for improving sanitation. The health sector now needs its commitment and leadership to help achieve a world in which everybody has access to adequate sanitation.
This programme will provide candidates the basics of sanitation and health and helps them to understand & evaluate the various programmes related to health & sanitation so that they cantake measures to maintain and improve standard of health and help the society to move towards better health & sanitation which is a need of today’s world.
Programme Modules
International Affiliation with
Programme Modules
International Affiliation with
Module 1: Public Health: Scientific, Political and Economic Aspects
Module 2: Policies & Programmes for Public Health
Module 3: Epidemiology: Principles, Methods and limitations
Module 4: Epidemiology & Its Principles
Module 5: Concept of Health & Disease
Module 6: Screening of Diseases
Module 7: Environmental Health
Module 8: Infection Control
Module 9: Nutrition & Health
Module 10: Food Sanitation & Food Chemistry
Module 11: Waste Management
Module 12: Water & Environmental Sanitation
Eligibility
10 + 2.
Programme Duration
The duration to complete this programme is 1 year.
Registration
The registration dates for this programme run by the Institute are updated timely on the webpage. Effective online learning tools incorporated into the design of the webpage make the programme lectures, online live classes and study material easily accessible. This gives a huge window of self-regulated and self-paced performance to the participants.
Programme Deliverables
A comprehensive study material for all the modules in hard copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
- Assignments for all the programme modules for continuous evaluation and guidance.
- Interactive or online live sessions on all key areas of the programme giving all flexibility to the participants.
- Online classes for all the modules will be conducted on the weekends. Moreover, a doubt clearing session will also be scheduled before the examination
- All the efforts are made by IGMPI faculty members to make the entire programme modules easily understandable.
- Assessment and evaluation for all the programme modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
- At the end of each programme modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
- All learning and training delivery initiatives shall be conducted in English.
Examination and Certification
All the participants are obliged to timely submit completed assessment assignments (during the programme, usually after every module) and appear for an online exam at the end of the programme. After successful completion, the participants will receive a certificate of Diploma in Health & Sanitation (DHSI), IGMPI. For all the above-mentioned modules, Online Classes (Online mode) or face-to-face classes (Regular mode), elaborate programme material, self-assessment assignments would be provided by the Institute. Details get updated on the webpage as well.
Placement Assistance & Corporate Relations
The Institute has partnered with many organizations for providing with placement assistance to in its participants. Besides, it has a robust placement cell comprised of senior level Human Resources professionals and Talent Acquisition experts which maintains close links with business and industry. This cell is continuously engaged in promoting the employability of our participants and encouraging the concerned Human Resources department and Hiring Managers to recruit/hire our participants for their vacant positions. The efforts of our placement cell also include helping with professional resume writing & interview skills.
In recent months the Institute has witnessed more and more participation from professionals working with global healthcare like Big 4, Accenture, SGS, Fortune 100, Uniqus Consultech Ltd., ESG Book, Vision 360, TCS, Ckinetics, CSE(Centre for Science and Environment, TERI (The Energy and Research Institute), Iforest, CEEW (Council on Energy, Environment and Water ), CPR (Centre for Policy and Research ), Centre for Energy and Environment Sustainability, WWF (Worl wildlife Fund ), IWMI (International Water Management Institute ), Greenpeace, 350.org, ATREE, Chintan Environmental Research and Action Group, Sankalp Taru Foundation, TBS, Tarun Bharat Sangh, Mukti.
Future career prospects
Many opportunities in Government, private and NGOs sectors viz. government public health departments/ railways/ municipal corporations/ airports / town panchayat and industries. The qualified professionals can work as Health Inspectors, Sanitary Inspectors and Public Health Workers in the above-mentioned departments. Candidates can also get opportunities to work in various programmes run by government like Swach Bharat Abhiyan, Pradhan Mantri Swasthya Suraksha Yojana, National Health Mission, National Vector Borne Disease Control Program, Central Rural Sanitation Programme, Total Sanitation campaign etc.
Diploma in Industrial Hygiene (DIH)
Industrial hygiene is the science of guarding the health and safety of people at their workplace. The processes of industrial hygiene involve identifying, analysing, and controlling workplace conditions and hazards to prevent dangerous and harmful work environments. The COVID-19 pandemic has highlighted the importance of industrial hygiene and made it more important than ever. Especially as businesses reopen and people re-enter the workforce, industrial hygienists are needed to help, identify and mitigate risks and hazards. Even before the pandemic, however, industrial hygienists had been applying their scientific skills to promote health standards and help to a safer global workforce. For those interested in this field, it is important to understand exactly what industrial hygiene is and how an advanced course such as IGMPI’s Diploma in Industry Hygiene can contribute to develop professionals for impactful careers in the wake of COVID-19 and beyond.
Programme Modules
International Affiliation with
Programme Modules
International Affiliation with
Module 1: Introduction to Human Physiology and Industrial Diseases
Module 2: Basic Principles of Occupational Hygiene
Module 3: OSHA and Industrial Hygiene
Module 4: Identifying, measuring and analysing workplace health hazards and exposures (chemical, physical, biological, ergonomic)
Module 5: Vibration and Noise
Module 6: Radiation and Thermal Environment
Module 7: Asbestos and other Fibres
Module 8: Controlling Risks to Health
Module 9: Participatory Approaches for the Improvement of Working Conditions
Module 10: Case Studies
Module 11: Capstone Project
Eligibility
10 + 2.
Programme Duration
The duration to complete this programme is 1 year.
Registration
The registration dates for this programme run by the Institute are updated timely on the webpage. Effective online learning tools incorporated into the design of the webpage make the programme lectures, online live classes and study material easily accessible. This gives a huge window of self-regulated and self-paced performance to the participants.
Programme Deliverables
A comprehensive study material for all the modules in hard copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
- Assignments for all the programme modules for continuous evaluation and guidance.
- Interactive or online live sessions on all key areas of the programme giving all flexibility to the participants.
- Online classes for all the modules will be conducted on the weekends. Moreover, a doubt clearing session will also be scheduled before the examination
- All the efforts are made by IGMPI faculty members to make the entire programme modules easily understandable.
- Assessment and evaluation for all the programme modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
- At the end of each programme modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
- All learning and training delivery initiatives shall be conducted in English.
Examination and Certification
All the participants are obliged to timely submit completed assessment assignments (during the programme, usually after every module) and appear for an online exam at the end of the programme. After successful completion, the participants will receive a certificate of Diploma in Industrial Hygiene, IGMPI. For all the above-mentioned modules, Online Classes (Online mode) or face-to-face classes (Regular mode), elaborate programme material, self-assessment assignments would be provided by the Institute. Details get updated on the webpage as well.
Placement Assistance & Corporate Relations
The Institute has partnered with many organizations for providing with placement assistance to in its participants. Besides, it has a robust placement cell comprised of senior level Human Resources professionals and Talent Acquisition experts which maintains close links with business and industry. This cell is continuously engaged in promoting the employability of our participants and encouraging the concerned Human Resources department and Hiring Managers to recruit/hire our participants for their vacant positions. The efforts of our placement cell also include helping with professional resume writing & interview skills.
In recent months, the Institute has witnessed more and more participation from professionals working with global, healthcare like Big 4, Accenture, SGS, Fortune 100, Uniqus Consultech Ltd., ESG Book, Vision 360, TCS, Ckinetics, CSE(Centre for Science and Environment, TERI (The Energy and Research Institute), Iforest, CEEW (Council on Energy, Environment and Water ), CPR (Centre for Policy and Research ), Centre for Energy and Environment Sustainability, WWF (Worl wildlife Fund ), IWMI (International Water Management Institute ), Greenpeace, 350.org, ATREE, Chintan Environmental Research and Action Group, Sankalp Taru Foundation, TBS, Tarun Bharat Sangh, Mukti.
Future career prospects
Industrial hygienists are qualified professionals who “predict, recognize, evaluate, and recommend controls for environmental and physical hazards that can affect the health and well-being of workers,”. They also responsible help organizations to understand and adhere to federal, state and local safety regulations. Industrial hygienists can help many types of organizations, such as government agencies, labor unions and educational institutions. The largest employers of occupational health and safety specialists, including industrial hygienists, were Government organizations, Manufacturing companies, Construction companies, Scientific and technical consulting services etc.
Advisory Board and team
Our Advisory Board Members : https://igmpi.ac.in/our-advisors-and-team

Institute of Good Manufacturing Practices India

C-6, Qutab Institutional Area, Near Old JNU Campus, New Delhi–110016, India.
18001031071
(Toll Free -9:00 am to 5:30 pm IST-except on Central Government holidays)/
info@igmpi.ac.in