Trusted by organization & traning participants in over 45 countries
Traning | Certification | Education | Research
Prospectus
Academic Year 2025-26 (Session January-February, 2026)
Post Graduate Diploma/Executive Diploma in Pharmaceutical Business Management (PGDPBM/EDPBM)
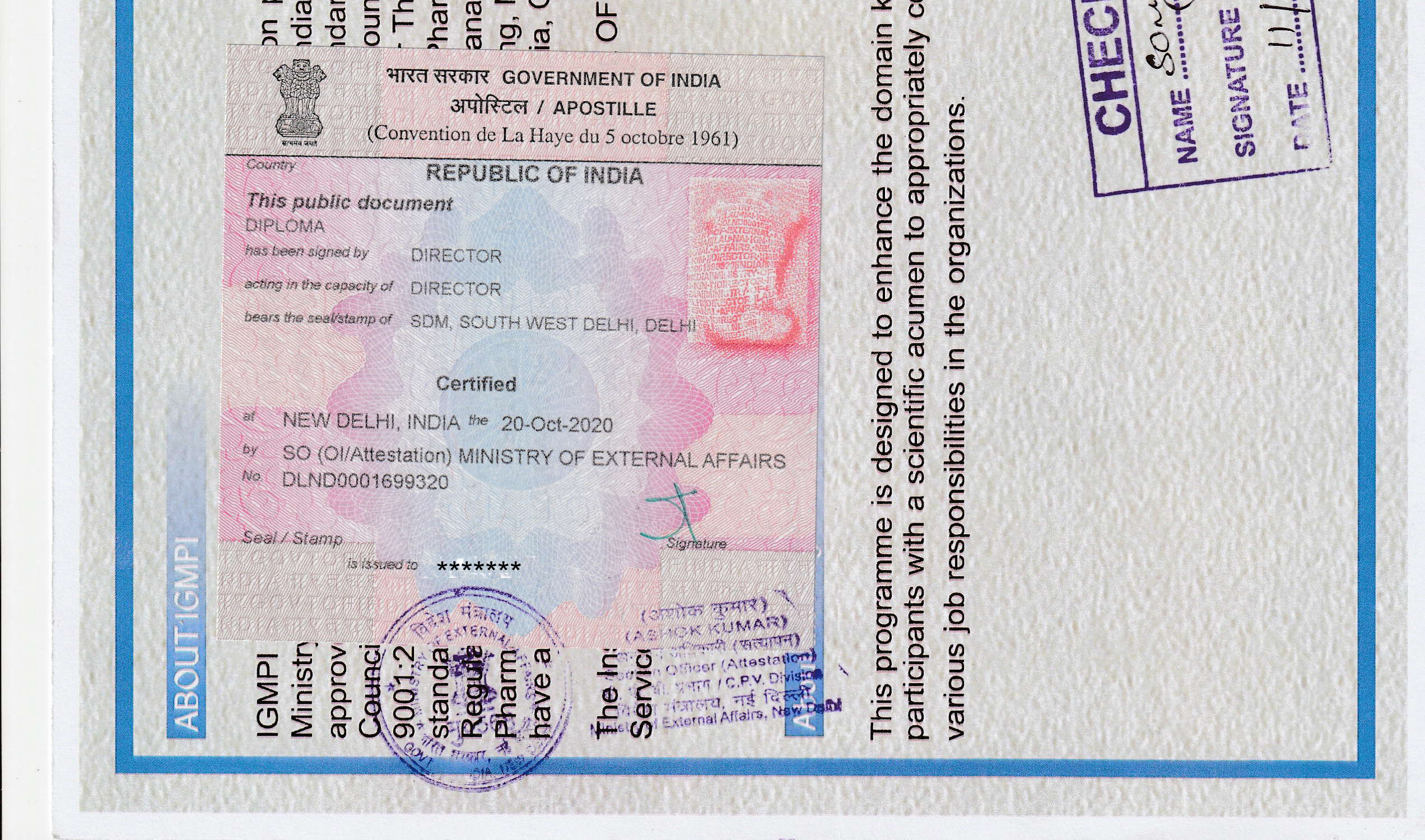
igmpi.ac.inAbout IGMPI
The Institute of Good Manufacturing Practices India, an autonomous Institute recognised by Ministry of Commerce & Industry, accredited Vocational Institution of Ministry of Education, Government of India and approved by Food Safety and Standards Authority of India (FSSAI), presents a unique, friendly and interactive platform to get rid of all your GMP compliance related issues. GMP—an essential element of industries like pharmaceutical, cosmetic, Ayurveda, biotech, homeopathic, medical device and food manufacturing and sustainability services—in itself is the most dynamic part which witnesses frequent changes in terms of new rules being added and old ones being renewed. Thus, keeping oneself updated with current GMPs is essential to remain aligned with evolving industry requirement and standards.
Our team comprises knowledgeable professionals from diverse sectors such as Pharma, Healthcare, Food, Nutraceutical, and Nutrition industries, pooling together their expertise, know-how, and experiences to create this GMP guide. IGMPI is moving hand in hand with technology advances and has gained recognition as a stronger and better education and training platform provider for professionals and students in the areas of GMP, Quality Assurance and Control, Pharma, Food & Nutrition, Environment and Healthcare Regulatory Affairs, Clinical Research, Pharmaceutical IPR, Good Laboratory Practice and Product Management. Keeping pace with the digital era, IGMPI has also expanded into the domains of Geoinformatics, Remote Sensing, Cybersecurity, Information Security, and Network Security, preparing learners to address the growing need for secure and resilient information systems as well as advanced geospatial solutions.
Our board of governors and specialists have collated their acumen and are offering state-of-the-art courses which include GMP training, Quality Assurance and Control, Pharma and healthcare Regulatory Affairs, Clinical Research, Pharmaceutical IPR, Environment Social Governance (ESG), Good Laboratory Practice, Information Security Compliance and Remote Sensing. These are delivered in the form of formal classroom studies, online/interactive programmes, online seminars, and onsite training programmes, along with insights into global industry practices. In short, a round-the-clock service is provided for any information in these areas required by anybody from around the country and abroad.
Based on high standards of quality, the training programmes in Pharma, Healthcare and Food GMP, Quality Assurance and Quality Control, Regulatory Affairs, IPR, Pharma Product Management, Public Health, Hospital Management, Clinical Research, Pharmacovigilance, Medical Writing, Medical Coding, Nanotechnology, Drug Design and Discovery, Food QA&QC have been approved by the Quality Council of India, which is an autonomous body and an accreditation authority for education & vocational training providers under the Ministry of Commerce & Industry, Government of India. IGMPI is duly licensed and certified by Bureau of Indian Standards (BIS) under Bureau of Indian Standards (Conformity Assessment) Regulations 2018.
Accreditation and Awards
The Institute of Good Manufacturing Practices India, an autonomous Institute recognised by the Ministry of Commerce & Industry, Government of India is duly licensed and certified by Bureau of Indian Standards (BIS) under Bureau of Indian Standards (Conformity Assessment) Regulations 2018 (Active license number: ERO/EOMSM/L-8000027) for offering education and training programmes in the sectors of Pharmaceutical, Food & Nutrition, Healthcare, Environmental Health & Safety, Environmental Social and Governance (ESG), Psychology, Medical Devices, Cosmetics, Remote Sensing, Health Insurance, Product & Marketing Management, Cybersecurity, Fire Safety, and Renewable Energy in the regular Diploma (full or part-time) and online modes.


IGMPI is an accredited Vocational Institution (AVI no. 710367) of the Ministry of Education, Government of India. Institute of Good Manufacturing Practices India is Management System certified under NABCB accreditation. IGMPI is also approved by Food and Life Sciences Sector Skill Councils recognized by the National Council of Vocational Education and Training (NCVET).

The Post Graduate and Executive Diploma programmes of IGMPI in Good Manufacturing Practices, Regulatory Affairs, Intellectual Property Rights, Quality Assurance and Quality Control, Public Health, Nanotechnology, Hospital Management, Product Management, Sales and Marketing Management, Clinical Research, Medical Writing, Drug Discovery and Development, Pharmacovigilance, Medical Coding have been duly assessed and approved by Quality Council of India, Government of India based on fulfillment of QCI's following criteria:
1. Course Content
2. Course Design
3. Course Material
4. Instructors
5. Class size & Attendance
6. Facilities
7. Evaluation of Students
8. Written Examination
9. Certificate
IGMPI is also approved by Food Safety and Standards Authority of India (FSSAI) (FSSAI ID: TPINS18). IGMPI® is licensed by Department of Food Safety & Drug Administration under the Drugs and Cosmetics Act, 1940 and registered under Food Safety and Standards Act 2006.
IGMPI is also approved by Sector Skills Council under National Skill Development Corporation (NSDC) setup by Ministry of Skill Development & Entrepreneurship, Govt of India (TC ID:TC342267).
IGMPI has been conferred with the prestigious "BUREAU OF INDIAN STANDARDS (BIS) AWARD OF HONOUR 2023". QUALITY COUNCIL OF INDIA (QCI) has also conferred IGMPI with D.L. SHAH NATIONAL QUALITY AWARD, Certificate of Merit & ASSOCHAM has conferred IGMPI with the Services Excellence Award based on excellence of its services to the students and training participants.
IGMPI's Membership with IPA
IGMPI is a Lifetime Institutional member of Indian Pharmaceutical Association (IPA).

Bureau of Indian Standards (BIS)
Bureau of Indian Standards (BIS) came into existence through an act of Parliament in 1987. BIS is the National Standard Body of India established under the BIS Act 2016 for the harmonious development of the activities of standardization, marking and quality certification of goods and for matters connected therewith or incidental thereto. The Bureau is a Body consisting of 25 members representing both Central and State governments, Members of Parliament, industry, scientific and research institutions, consumer organizations and professional bodies; with Union Minister of Consumer Affairs, Food and Public Distribution as its President and with Minister of State for Consumer Affairs, Food and Public Distribution as its Vice-President.
Quality Council of India (QCI)
Quality Council of India is set up by the Government of India to establish and operate national accreditation structure and promote quality through National Quality Campaign. QCI is registered as a non-profit society with its own Memorandum of Association. QCI is governed by a Council of 38 members and Chairman of QCI is appointed by the Prime Minister on recommendation of the industry to the government. The Department of Industrial Policy & Promotion, Ministry of Commerce & Industry, is the nodal ministry for QCI.
International Recognition
For providing its education and training services to overseas students, IGMPI is registered with the Directorate General of Foreign Trade, Government of India and our Export Import Code is AADCI7680Q.
IGMPI is an Institutional Member of the International Society for Quality in Health Care

Bureau of Indian Standards (BIS) is a member of International Organization for Standardization (ISO) and through the Indian National Committee (INC) which is a member of International Electrotechnical Commission (IEC). BIS is also a member of regional standards bodies like Pacific Area Standards Congress (PASC) and South Asian Regional Standards Organization (SARSO). India started taking part in IEC from 1911 and subsequently the then Indian Standards Institution (now BIS) took over the responsibility of Indian National Committee of IEC(INC-IEC) in 1949. Since then the INC-IEC is actively participating in the activities of the IEC both at the policy level and technical work and carrying out the responsibilities as member body of IEC Council. India is a member in Standards Management Board (SMB) of IEC since 2015.BIS has also signed Bilateral Cooperation Agreements (BCA)/Mutual Recognition Agreements (MRA) with the National Standards Bodies of several countries like Afghanistan, Bangladesh, Belarus, Egypt, European Union , Germany, Ghana, Greece, Indonesia, Iran, Japan, Jordon, Kenya, Kyrgyzstan, Mali, Mauritius, Nigeria, Russia, Saudi Arabia , Slovakia, Slovenia, Suriname, USA, UAE, Uzbekistan, Viet Nam, Bhutan, Brazil, Israel, Nepal, Pakistan and Sri Lanka.
National Accreditation Board for Certification Bodies (NABCB), Quality Council of India is a member of International Accreditation Forum (IAF) & Pacific Accreditation Cooperation (PAC) as well as signatory to its MLAs for Quality Management Systems, Environmental Management Systems and Product Certification. NABCB is also a Full Member of International Laboratory Accreditation Cooperation (ILAC) & Asia Pacific Laboratory Accreditation Cooperation (APLAC) as well as signatory to its MRAs for Inspection.
Post Graduate Diploma/Executive Diploma in Pharmaceutical Business Management (PGDPBM/EDPBM)
Pharmaceutical Business Management is a specialized field that blends pharmaceutical sciences with core business strategies to meet the dynamic demands of the healthcare and pharmaceutical industries. The curriculum typically includes subjects such as pharmaceutical marketing, drug regulations, supply chain management, healthcare economics, and strategic management. Designed to develop both scientific understanding and managerial acumen, the program prepares students for a wide range of careers, including product management, regulatory affairs, pharmaceutical sales, and marketing analytics. Professionals in this field play a crucial role in ensuring that pharmaceutical products are developed, marketed, and delivered efficiently and ethically, bridging the gap between scientific innovation and market needs.
The performance of a product, the strength of a brand, and even the reputation of a company often hinge on the effectiveness of its business team. Given the significant influence these professionals wield, it becomes imperative to have skilled and well-informed individuals driving these functions. Recognizing the critical importance of this role, the Pharmaceutical Business Managementat IGMPI has been thoughtfully designed to equip future professionals with the expertise and insight required to navigate and excel in this demanding and impactful domain.
Programme Structure
International Affiliation with
Programme Structure
International Affiliation with
Module 1: Introduction to Pharmaceutical Business Management
Module 2: Principles of Management in Pharmaceutical Business
Module 3: Strategic Pharmaceutical Marketing and Market Segmentation
Module 4: Pharmaceutical Sales & Advertising Management
Module 5: Guidelines of Visual Aids and Input Designing
Module 6: Pharmaceutical Supply Chain and Logistics Management
Module 7: Fundamentals of Drug Discovery and Development Processes
Module 8: Healthcare System in US, India and global perspectives
Module 9: Pharmaceutical Business Models and Market Intelligence
Module 10: Pharmacoeconomics and Drug Pricing Regulations
Module 11: Legal and Regulatory Framework of Pharmaceutical Industry
Module 12: Promotional Strategies and Communication Models in Pharma
Module 13: Professional and Business Communication in the Pharmaceutical Industry
Module 14: Digital and Relationship marketing –KOL and CRM
Module 15: E-Commerce and Technology Integration in Pharmaceutical Business
Module 16: Entrepreneurship and Innovation in Pharmaceutical Industry
Module 17: Industry Based Case Studies and Real-World Business Applications
Module 18: Capstone Project
Eligibility
Graduates in any discipline are eligible for our Post Graduate Diploma, Executive Diploma and Professional Certification Programmes.
Programme Duration
The minimum duration to complete the PG diploma programme is 12 months and maximum is 24 months. The minimum duration to complete the executive diploma programme is 6 months and maximum is 12 months.
Programme Mode
Registrations are currently open for regular and Part-time (Online Live Classes) both modes.
Programme Deliverables
A comprehensive study material for all the modules in hard copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
- Assignments for all the programme modules for continuous evaluation and guidance.
- Interactive or online live sessions on all key areas of the programme giving all flexibility to the participants.
- Part-time (Online Live Classes) for all the modules will be conducted on the weekends. Moreover, a doubt clearing session will also be scheduled before the examination.
- All the efforts are made by IGMPI faculty members to make the entire programme modules easily understandable.
- Assessment and evaluation for all the programme modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
- At the end of each programme modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
- All learning and training delivery initiatives shall be conducted in English.
Examination & Certification
All the participants are obliged to timely submit completed assessment assignments (during the programme, usually after every module) and appear for an online exam at the end of the programme. After successful completion, the participants will be awarded Post Graduate Diploma/Executive Diploma in Pharmaceutical Business Management by IGMPI. For all the above-mentioned modules, Part-time (Online Live Classes) or face-to-face classes (Regular mode), elaborate programme material, self-assessment assignments would be provided by the Institute. Details get updated on the webpage as well.
Discipline in Classes and Examination
Every student is required to observe a disciplined behaviour during her/his classes, assessments & examinations and to follow instructions from the Professors. Any act of indiscipline may result into discredit & it will be mentioned in her/his academic report.
Placement Assistance & Corporate Relations
The Institute has partnered with many organizations for providing with placement assistance to its participants. Besides, it has a robust placement cell comprised of senior level Human Resources professionals and Talent Acquisition experts which maintains close links with business and industry. This cell is continuously engaged in promoting the employability of our participants and encouraging the concerned Human Resources department and Hiring Managers to recruit/hire our participants for their vacant positions. The efforts of our placement cell also include helping with professional resume writing & interview skills.
In recent months the Institute has witnessed more and more participation from professionals working with global pharmaceuticals like Dr. Reddy’s Laboratories, Cipla, Cadila Healthcare, Gland Pharma, Aurobindo Pharmaceuticals, Lupin, Torrent Pharmaceuticals, Zydus Lifesciences, Accenture, Tata Consultancy Services, Bayer, Galentic Pharma (India) Pvt. Ltd., Wipro, Genpact, Emcure, Lupin, Mankind, Johnson & Johnson, Jubilant Lifesciences, Roche, Zydus, Sun Pharmaceuticals, Lupin, Eli Lilly & Company, Pfizer, Merck, Astrazeneca, Glenmark, Alembic, Hetero, Thermo, Lakme, Nivea, Oriflame, Loreal, Lotus, etc. The IGMPI’s Corporate Resource Division actively recommends our students and training participants for various job requirements and specialized roles to Human Resource, Talent Acquisition as well as the heads of various departments in Pharmaceutical, Healthcare industries on regular basis.
Future career prospects
Training in Pharmaceutical Business Management opens up a wide array of career opportunities in the rapidly growing and evolving pharmaceutical and healthcare sectors. Graduates of this discipline are well-equipped to take on strategic roles that combine scientific knowledge with business acumen. Some of the promising career paths include:
- Product Management: Overseeing the lifecycle of pharmaceutical products, from development to market launch and beyond.
- Pharmaceutical Sales and Marketing: Driving brand strategy, market penetration, and customer engagement through targeted campaigns.
- Regulatory Affairs: Ensuring compliance with global and national pharmaceutical regulations and facilitating product approvals.
- Market Research and Analytics: Gathering and interpreting data to guide business strategies and decision-making.
- Supply Chain and Distribution Management: Coordinating the efficient movement of products from manufacturing to end users.
- Business Development and Licensing: Identifying growth opportunities through partnerships, licensing deals, and market expansion.
- Healthcare Consulting: Advising pharmaceutical firms on strategy, operations, and policy implications.
As the global demand for innovative healthcare solutions grows, the need for skilled professionals who can navigate both the scientific and commercial landscapes of the pharmaceutical industry continues to rise. This training positions candidates to not only enter the industry but also advance rapidly into leadership and decision-making roles.
Programme Fee Details
| S.No. | Programmes | Duration | Mode | Programmes Fee |
|---|---|---|---|---|
| 1 | Under Graduate Diploma | 12 months | Part-time (Online Live Classes) | Rs.58,000/- (USD 1400) |
| 2 | Post Graduate Diploma | 12 months | Part-time (Online Live Classes) | Rs.78,000/- (USD 1600) |
| 3 | Executive Diploma | 6 Months | Part-time (Online Live Classes) | Rs.58,000/- (USD 1400) |
Advisory Board and team
Our Advisory Board Members : https://igmpi.ac.in/our-advisors-and-team
Institute of Good Manufacturing Practices India

C-6, Qutab Institutional Area, Near Old JNU Campus, New Delhi–110016, India.
18001031071
(Toll Free -9:00 am to 5:30 pm IST-except on Central Government holidays)/
info@igmpi.ac.in