

Centre for Nutrition and Dietetics Studies (CNDS) has been set up under the aegis of IGMPI registered as a non-profit society (under The Societies Registration Act, 1860) with the Government of India.The Centre is committed to promote proper diet and nutrition which is crucial to the health and happiness of the society at large.
CNDS is imparting education in the field of Nutrition and Dietetics to thousands of knowledge seekers in the classroom setting as well as research dissertation, field and online learning training programmes.
CNDS provides and education programmes and imparts extensive knowledge through classroom and online modes, in the holistic Nutrition programmes like Nutrition and Dietetics as well as specialized courses like diabetes education, paediatric nutrition, nutritional oncology, ayurvedic food and nutrition, obesity and weight management, clinical diabetology, nutritional gastroenterology and hepatology and many other programmes. Our courses are designed with outmost care given to the industry requirements and shaping careers in the field of Nutrition dietetics, medical and non-medical.
Students gets to experience the expertise from faculties who are specialized in Nutrition, dietetics, foods, pharmaceuticals, healthcare, etc. The centre is well equipped with all the teaching aids and advance laboratory equipment and devices.
The Centre is committed to making nutrition accessible to everyone through nutrition recommendations, easy-to-follow explanations, interactive two way communication lectures, dynamic presentations of the lectures.Apart from this the center provides ample opportunities for the students to comprehend the latest diet plans and nutrition requirements with hands-on practices in Industry, Hospitals and Clinics.
Post completion of the all the courses, CNDS also provides job and Internship placement assistance to students in an easy and effortless way.
Institute of Good Manufacturing Practices India, registered as a non-profit society (under The Societies Registration Act,1860) with Government of India recognised by Ministry of Commerce & Industry, Government of India, accredited Vocational Institution of Ministry of Education, Government of India and approved by Food Safety and Standards Authority of India (FSSAI) presents unique, friendly and interactive platform to get rid of all your GMP related glitches. GMP- is an essential element of industries like pharmaceutical, cosmetic, Ayurveda, biotech, homeopathic, medical device and food manufacturing. GMP in itself is the most dynamic part which witnesses frequent changes in terms of newer rules being added and older ones being renewed. Keeping self updated with current GMPs thus becomes inevitable to stay abreast with the changing industry needs and practices.
Our group of learned professionals from above mentioned sectors of the Pharma, Healthcare and Food industries have put together their knowledge; know about and practical experiences in form of this GMP guide. IGMPI is moving hand in hand with technology advances and has gained recognition as a stronger and better training platform provider for professionals and students in the areas of GMP, Quality Assurance and Control, Pharma, Food and healthcare Regulatory Affairs, Clinical Research, Pharmaceutical IPR and Good Laboratory Practice and Product Management. The importance of quality healthcare and foods is known to our founders and thus numerous efforts are being made to offer friendly but effective and easy regular and online/online sources of GMP training, Quality Assurance and Control, Pharma and healthcare Regulatory Affairs, Clinical Research, Pharmaceutical IPR and Good Laboratory Practice in form of formal classroom studies, online/interactive programmes, online seminars, as well as onsite training programmes along with knowledge of worldwide affairs of the industry; in short a round-the-clock help for any information in these areas needed by anybody from around the world. Based on high standard of quality, the training programmes in Pharma, Healthcare and Food GMP, Quality Assurance and Quality Control, Regulatory Affairs, IPR, Pharma Product Management, Public Health, Hospital Management, Clinical Research, Pharmacovigilance, Medical Writing, Medical Coding, Nanotechnology, Drug Design and Discovery, Food QA&QC etc areas have been approved by Quality Council of India, which is an autonomous body and an accreditation authority for education & vocational training providers under the Ministry of Commerce & Industry, Government of India.
The IGMPI's team of technology experts and other Industry advisors together pursue to make cGMP knowledge, training in the area of Pharma and Food manufacturing easily accessible, through this platform.
IGMPI is recognized by the Ministry of Commerce & Industry Government of India. Duly licensed and certified by Bureau of Indian Standards (BIS) under Bureau of Indian Standards (Conformity Assessment) Regulations 2018 (License number: CRO/QM/L-8004228) for offering education and training programmes in the areas of Pharmaceutical, Food, Nutrition and Healthcare.


Institute of Good Manufacturing Practices India (IGMPI) is registered as a non-profit society with its own Memorandum of Association and bye-laws under The Societies Registration Act, 1860, Government of India. IGMPI is an accredited Vocational Institution of Ministry of Education, Government of India.
The Post Graduate and Executive Diploma programmes of IGMPI in Good Manufacturing Practices, Regulatory Affairs, Intellectual Property Rights, Quality Assurance and Quality Control, Public Health, Nanotechnology, Hospital Management, Product Management, Sales and Marketing Management, Clinical Research, Medical Writing, Drug Discovery and Development, Pharmacovigilance, Medical Coding have been duly assessed and approved by Quality Council of India, Government of India based on fulfillment of QCI's following criteria:
IGMPI is also approved by Food Safety and Standards Authority of India (FSSAI) (FSSAI ID: TPINS18). IGMPI® is licensed by Department of Food Safety & Drug Administration under the Drugs and Cosmetics Act, 1940 and registered under Food Safety and Standards Act 2006. QUALITY COUNCIL OF INDIA (QCI) has also conferred IGMPI with D.L. SHAH NATIONAL QUALITY AWARD, Certificate of Merit & ASSOCHAM has conferred IGMPI with the Services Excellence Award based on excellence of its services to the students and training participants.
IGMPI is a Lifetime Institutional member of Indian Pharmaceutical Association (IPA).

IGMPI is an Institutional member of The Association of Food Scientists and Technologists (India), (AFSTI).

Bureau of Indian Standards (BIS) came into existence through an act of Parliament in 1987. BIS is the National Standard Body of India established under the BIS Act 2016 for the harmonious development of the activities of standardization, marking and quality certification of goods and for matters connected therewith or incidental thereto. The Bureau is a Body consisting of 25 members representing both Central and State governments, Members of Parliament, industry, scientific and research institutions, consumer organizations and professional bodies; with Union Minister of Consumer Affairs, Food and Public Distribution as its President and with Minister of State for Consumer Affairs, Food and Public Distribution as its Vice-President.
For providing its education and training services to overseas students, IGMPI is registered with the Directorate General of Foreign Trade, Government of India and our Export Import Code is AADCI7680Q.
IGMPI is an Institutional Member of the International Society for Quality in Health Care 
Bureau of Indian Standards (BIS) is a member of International Organization for Standardization (ISO) and through the Indian National Committee (INC) which is a member of International Electrotechnical Commission (IEC). BIS is also a member of regional standards bodies like Pacific Area Standards Congress (PASC) and South Asian Regional Standards Organization (SARSO). India started taking part in IEC from 1911 and subsequently the then Indian Standards Institution (now BIS) took over the responsibility of Indian National Committee of IEC(INC-IEC) in 1949. Since then the INC-IEC is actively participating in the activities of the IEC both at the policy level and technical work and carrying out the responsibilities as member body of IEC Council. India is a member in Standards Management Board (SMB) of IEC since 2015.BIS has also signed Bilateral Cooperation Agreements (BCA)/Mutual Recognition Agreements (MRA) with the National Standards Bodies of several countries like Afghanistan, Bangladesh, Belarus, Egypt, European Union , Germany, Ghana, Greece, Indonesia, Iran, Japan, Jordon, Kenya, Kyrgyzstan, Mali, Mauritius, Nigeria, Russia, Saudi Arabia , Slovakia, Slovenia, Suriname, USA, UAE, Uzbekistan, Viet Nam, Bhutan, Brazil, Israel, Nepal, Pakistan and Sri Lanka.
National Accreditation Board for Certification Bodies (NABCB), Quality Council of India is a member of International Accreditation Forum (IAF) & Pacific Accreditation Cooperation (PAC) as well as signatory to its MLAs for Quality Management Systems, Environmental Management Systems and Product Certification. NABCB is also a Full Member of International Laboratory Accreditation Cooperation (ILAC) & Asia Pacific Laboratory Accreditation Cooperation (APLAC) as well as signatory to its MRAs for Inspection.
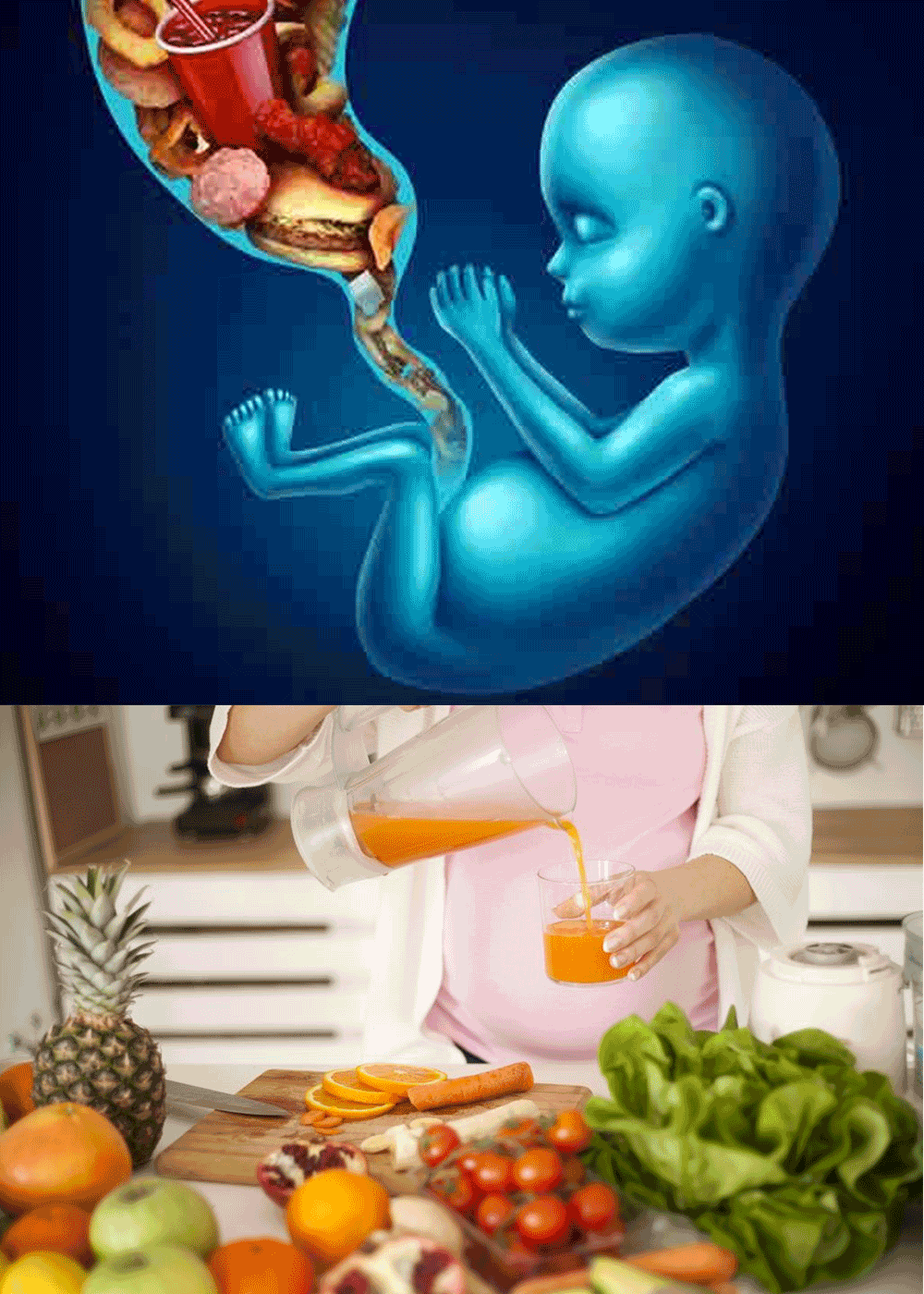
The Post Graduate Diploma/Executive Diploma in Nutritional Gynecology provides the essential knowledge and skills for the Life Sciences and Healthcare Professional like Dietitians, Doctors, Gynaecologist, Pharmacist, Pharmacologist etc.They will get a deeper insight of nutrition right from pregnancy till lactation.
The objective of this programme is to recognize nutrition and challenges in adopting these nutrition policies from the first trimester till third trimester, growth period of foetus, diseased conditions, preterm infants, breast feeding and complementary feeding. On completion of this programme students will be able to explain the nutritional requirements during different stages of pregnancy till lactation. They will also learn the nutritional requirements required for diseased condition like Gestational Diabetes, Rh incompatibility, Nausea, Back Pain, Depression and Anxiety, Foetal Problem, High Blood Pressure during pregnancy and lactation.
Centre for Nutrition and Dietetics Studies (CNDS), IGMPI has designed the programme keeping in mind practical approach related to Nutritional Gynecology. Students will have the chance to work on various case studies after completing all the modules in order to strengthen their hold for field work.
The programme has been designed for professionals and students aspiring to work in the field of Nutritional Gynecology as Dietitians, Nutritionists and Researchers.
Module 1: Introduction to Human Anatomy and Physiology
Module 2: Introduction to Nutritional Gynecology and understanding of female reproductive System
Module 3: Prevention of Feminine Nutritional deficiencies and Diet for Pre-Pregnancy
Module 4: Holistic Nutrition Approach during infertility and its Complications
Module 5: Physiology during Pregnancy
Module 6: Physiology during Lactation
Module 7: Diseases and Complications during Stage of Pregnancy
Module 8: Nutritional Requirements during Pregnancy
Module 9: Nourishment During Assisted Reproductive Technology
Module 10: Nutritional Requirement during Lactation and Breastfeeding
Module 11: Dietary Guidelines and Lifestyle Modification in Normal and Medical Conditions during Pregnancy and Lactation
Module 12: Introduction to Grabhsanskar and Ayurvedic Dietary Changes and its Significance
Module 13: Food Allergies during Pregnancy
Module 14: Myths of Foods Related during Pregnancy
Module 15: Nutritional Education and Counselling
Module 16: Practitioner Case Studies
Module 17: Practical Aspects of Different Stages during Pregnancy and Lactation
Any Graduate in Science/ Nutrition Science/ Food and Nutrition/ Pharma/ Medical/ Food Science & Technology/ Related disciplines & Candidates appearing for the final year of Bachelor's degree/ equivalent qualification exam or awaiting their results, are eligible to apply. Diploma holders are eligible for our Industry Certificate, and Certificate Programmes
The duration to complete this programme is 1 year (Post Graduate Diploma), 6 months (Executive Diploma).
The registration dates for this programme run by the Institute are updated timely on the webpage. Effective online learning tools incorporated into the design of the webpage make the programme lectures, study material and online classes easily accessible. This gives huge window of self-regulated and self-paced performance to the participants.
A comprehensive study material for all the modules in hard copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
-Assignments for all the programme modules for continuous evaluation and guidance
- Interactive or online live sessions on all key areas of the programme giving all flexibility to the participants.
- Online classes for all the modules will be conducted on the weekends. Moreover, a doubt clearing session will also be scheduled before the examination.
- All the efforts are made by IGMPI faculty members to make the entire programme modules easily understandable.
- Assessment and evaluation for all the programme modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
- At the end of each programme modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
- All learning and training delivery initiatives shall be conducted in English.
IGMPI follows a credit system based on all learning activities involved in studying for all PG Diploma Programmes. Each of your modules is equal to 4 credits. To successfully complete the programme, you will have to earn all the credits assigned to your programme.
All the participants are obliged to timely submit completed assessment assignments (during the programmes, usually after every module) and appear for an online exam at the end of the programmes. After successful completion, the participants will be awarded Post Graduate Diploma/Executive Diploma in Nutritional Gynecology by Center for Nutrition and Dietetics Studies (CNDS), IGMPI. For all the above-mentioned modules elaborate programme material, self-assessment assignments and project work details would be provided by the Institute from time to time. Details get updated on the webpage as well.
The Institute has partnered with many organizations for providing with placement assistance to in its participants. The robust placement cell comprises of senior level Human Resources professionals and Talent Acquisition experts which maintains close links with business and industry. We are engaged in promoting the employability of our participants by maintaining good rapport and relation with HR cell and recruiting managers of leading Food and Agriculture companies across the globe. The efforts of our placement cell also include helping with professional resume writing, interview skills & conducting mock interviews etc.
In recent Months the Institute has witnessed more and more participation from professionals working global healthcare and nutrition giants like Ministry of Health and Family Welfare, Fortis La Femme, Motherhood Hospital, Max Smart Super Specialty Hospital, Nanavati Hospital, MaxCure Suyosha Woman and Child Hospital, Titus Health Tech Pvt. Ltd., Amalth Lifecare Pvt. Ltd., Nutro Active Industries Pvt. Ltd., Essenzaa Nutrition, Insta Fitness Pvt. Ltd., MyHealthBuddy, Momkidcare, NutriSynapzz Therapeutix Pvt. Ltd., etc.
Centre for Nutrition and Dietetics Studies (CNDS), IGMPI's online programme is a professional programme targeted to cater the health industry needs trained health professionals. The information, guidance, practical training and off programme completion certificate will provide the participant with not one but many opportunities in the industry. This would come true in the form of job roles and positions like that of Gynecology Nutritionist, Dietitians, Maternal and Lactation Health Consultants, Position in Health Centres, and many more.


18001031071 (Toll Free), Phone: +91 11 26512850