(An Autonomous Body Recognized by Ministry of Commerce & Industry, Government of India)
Competency based placement focussed Education | Training | Research | Consultancy
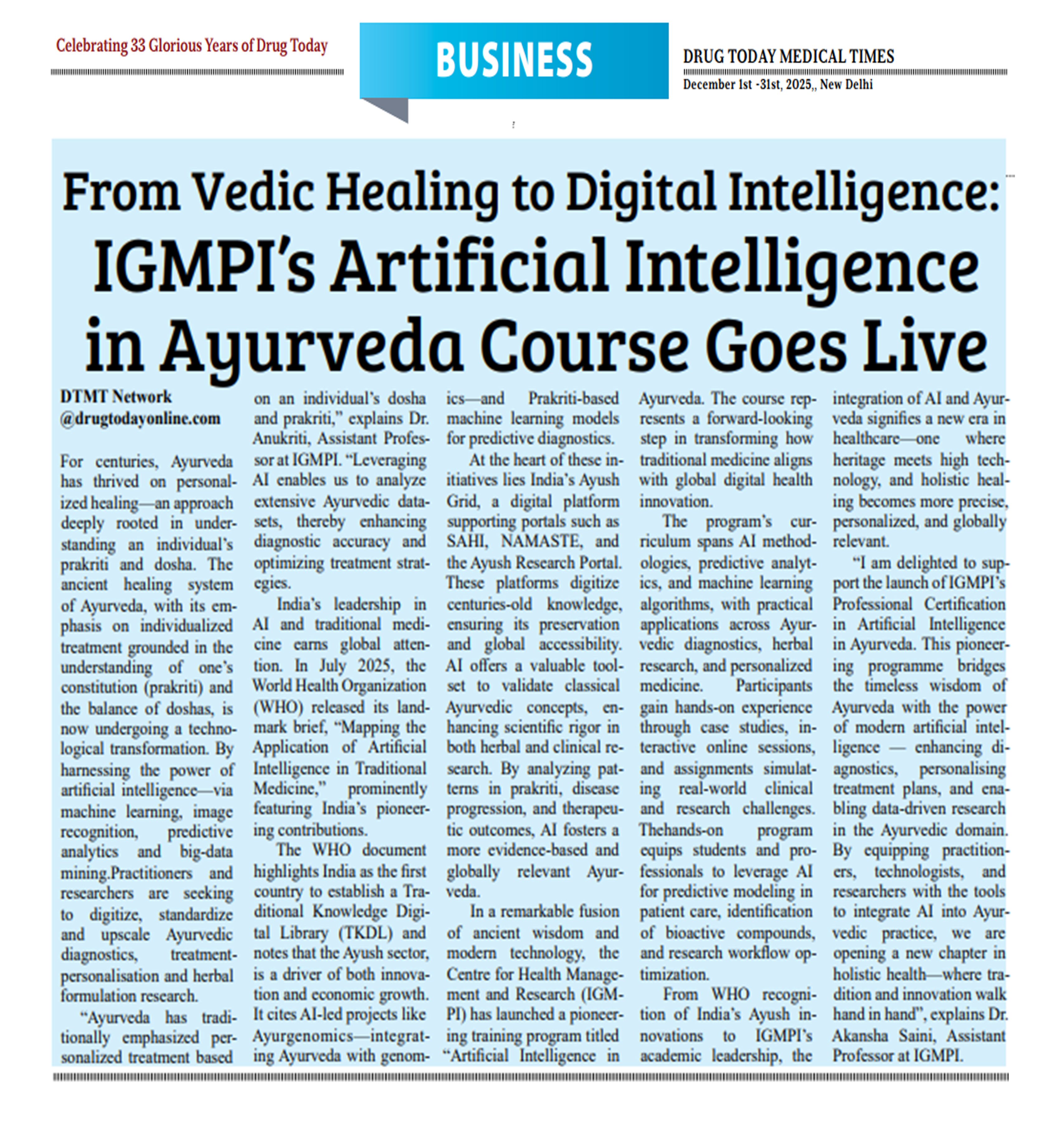
IGMPI in Media
Nutrition & Wellness Award 2025: CNDS, IGMPI is in Jury Panel
Nutrition & Wellness Award 2025: CNDS, IGMPI is in Jury Panel
Do You Always Compare Yourself to Others? Experts Explain How This Habit Harms Your Mental Health
Do You Always Compare Yourself to Others? Experts Explain How This Habit Harms Your Mental Health
IGMPI Director Dr. Syed S. Abbas Presents Prestigious Awards at ETNutriWell Conclave 2025
IGMPI Director Dr. Syed S. Abbas Presents Prestigious Awards at ETNutriWell Conclave 2025
National Education Day 2025: Why Health Education Is Essential For Girls At An Early Age
National Education Day 2025: Why Health Education Is Essential For Girls At An Early Age
Digital Forensics Strengthening Cybercrime Cases : Dr. Syed S Abbas, IGMPI
Digital Forensics Strengthening Cybercrime Cases : Dr. Syed S Abbas, IGMPI
Do You Feel Anxious Whenever You Sit Idle? Could You Be Suffering from Rest Anxiety?
Do You Feel Anxious Whenever You Sit Idle? Could You Be Suffering from Rest Anxiety?
Union Budget 2026–27: Industry leaders call for prevention-led healthcare and stronger public investment
Union Budget 2026–27: Industry leaders call for prevention-led healthcare and stronger public investment
Healthcare Leaders Flag Digital Security, GST Relief, AI Adoption Ahead of Union Budget 2026–27
Healthcare Leaders Flag Digital Security, GST Relief, AI Adoption Ahead of Union Budget 2026–27
Are We Eating Plastic Every Day? Shocking Facts About Microplastics in Food
Are We Eating Plastic Every Day? Shocking Facts About Microplastics in Food
A Psychologist Explains How Popcorn Brain Is Affecting Your Cognitive Skills
A Psychologist Explains How Popcorn Brain Is Affecting Your Cognitive Skills
IGMPI Launches Training Program to Address Documentation and Pharmacovigilance Gaps in Indian Pharma Sector
IGMPI Launches Training Program to Address Documentation and Pharmacovigilance Gaps in Indian Pharma Sector
How IGMPI is Shaping India’s Future Food Safety and Quality Workforce
How IGMPI is Shaping India’s Future Food Safety and Quality Workforce
We train professionals to see quality not as a checklist but as a mindset
We train professionals to see quality not as a checklist but as a mindset
IGMPI expands FoSTaC training support for India’s food businesses
IGMPI expands FoSTaC training support for India’s food businesses
IGMPI expands FoSTaC training support for India’s food businesses
IGMPI expands FoSTaC training support for India’s food businesses
Featured Article
Moderated by Shri Vinod Arora, Principal Advisor, IGMPI (Building FR&D Talent in India)
Moderated by Shri Vinod Arora, Principal Advisor, IGMPI
Moderated by Shri Vinod Arora, Principal Advisor, IGMPI
Moderated by Shri Vinod Arora, Principal Advisor, IGMPI
Fighting fakes with technology
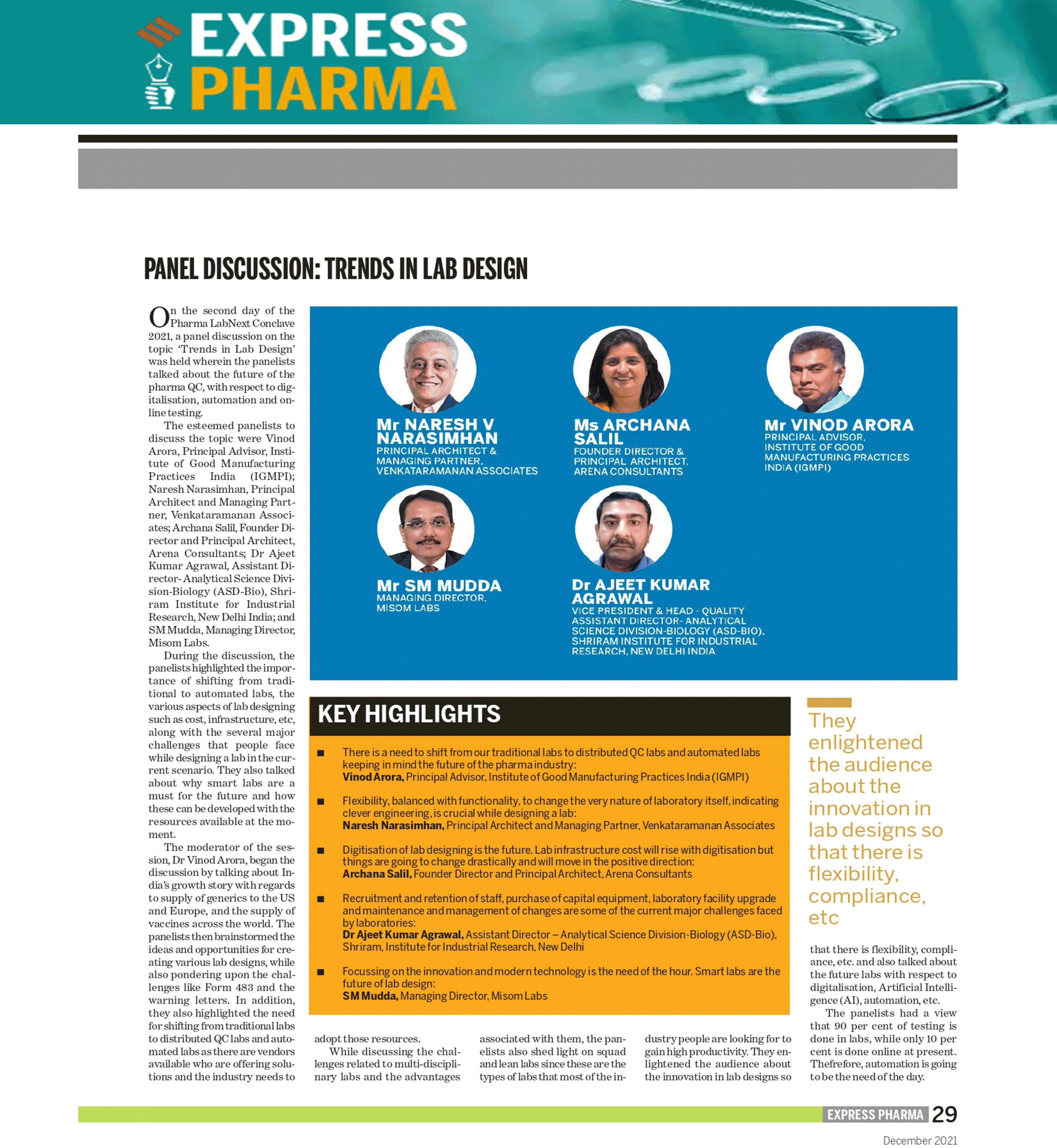
Trends in lab design
Oral solid dosage forms: Trends and opportunities
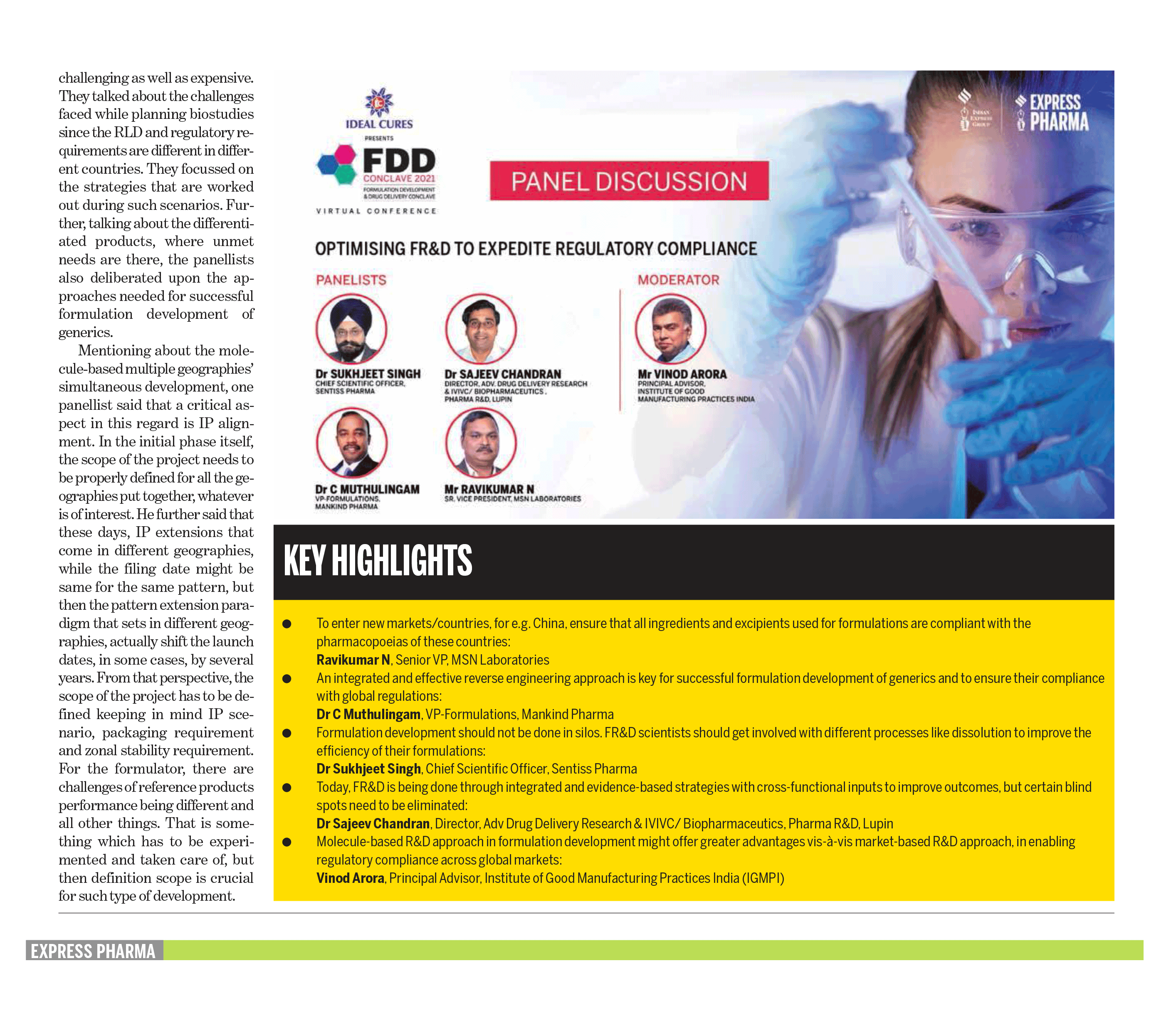
Optimising FR&D to expedite regulatory complaince
By Shri Vinod Arora, Principal Advisor, IGMPI