(An Autonomous Body Recognized by Ministry of Commerce & Industry, Government of India)
Competency based placement focussed Education | Training | Research | Consultancy
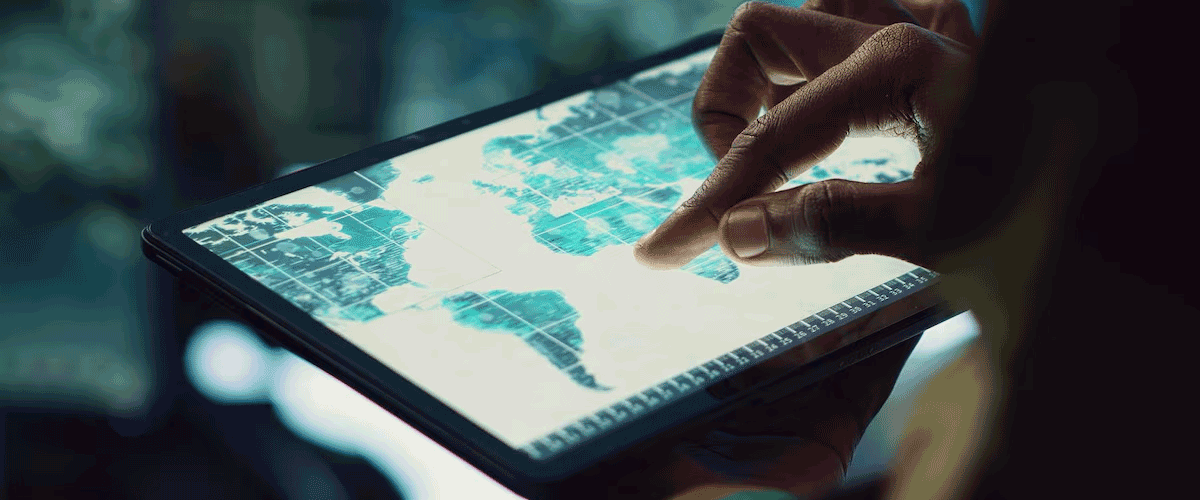
EMA Launches Interactive Clinical Trials Map for Europe
The European Medicines Agency (EMA) has launched an interactive map that allows users to explore clinical trial sites across the European Union. This new feature is part of the public Clinical Trials Information System (CTIS) portal and is designed to provide transparent, accessible information to patients, healthcare professionals, and researchers. The map displays detailed data on trial centers, investigators, and the status of clinical trials—including planned, ongoing, and completed studies.
Users can filter search results by location, trial phase, therapeutic area, and more, enabling them to quickly find relevant studies and contact information. The graphical, user-friendly interface was developed to make it easier for the public to engage with clinical research and support informed decision-making.
By centralizing and simplifying access to trial information, the EMA aims to boost public awareness, enhance patient participation, and promote collaboration across the clinical research ecosystem in the EU.
04-04-2025
📰 Recent News
- NPPA Clears Serum Institute to Stop Two Tetanus Toxoid Pack Sizes
- DoP Extends PLI Application Deadline for Meropenem and Ritonavir
- India Bans High-Dose Nimesulide Oral Formulations Over Safety Concerns
- India Proposes Prescription-Only Sale of Cough Syrups After Child Deaths
- FDA Publishes Revised Draft Guideline on Medical Gases
- FDA Issues Warning Letter to Cdymax India Pharma Over OOS and CAPA Failures
- FDA Highlights Data Integrity Risks of Excel in GMP Environments
- ICH Moves Toward Harmonised Biosimilar Development Guidelines
- FDA Criticism of Equipment Maintenance Under 21 CFR 211.67(a)
- GCP Duties for Reviewing Clinical Trial Insurance
