(An Autonomous Body Recognized by Ministry of Commerce & Industry, Government of India)
Competency based placement focussed Education | Training | Research | Consultancy
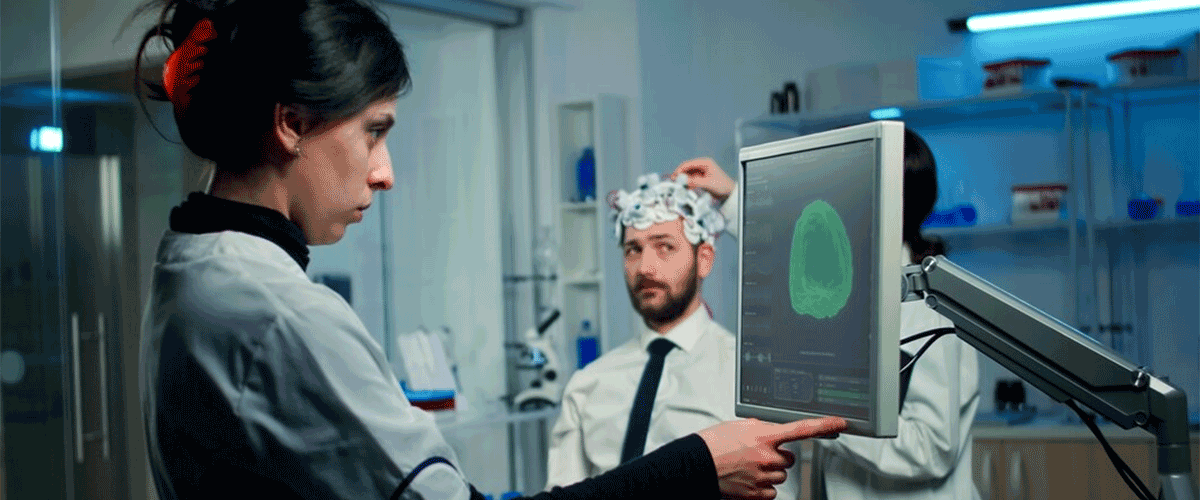
Precision Neuroscience receives FDA clearance for brain implant
Precision Neuroscience has received FDA 510(k) clearance for its Layer 7 Cortical Interface, a thin microelectrode array designed to map brain activity for up to 30 days. This clearance marks a key milestone for the New York-based startup, which is developing a brain-computer interface (BCI) system. The Layer 7 device rests on the surface of the cerebral cortex and is implanted through a small skull incision. It has been tested in 37 patients during temporary placements for procedures like brain tumor removal. With this FDA approval, the device can now be used for longer-term intraoperative brain mapping, enabling the company to collect high-resolution neural data. Precision plans to use this data to train algorithms for future BCI applications, such as controlling robotic limbs. The company is among the first to gain FDA clearance for a next-generation wireless BCI, positioning itself alongside competitors like Neuralink and Synchron, which has already begun human trials.
28-04-2025
📰 Recent News
- KORU Medical Seeks FDA Clearance to Expand FreedomEDGE into Oncology Infusions
- SleepRes Secures FDA Clearance for Adaptive KPAP Device to Treat Sleep Apnoea
- Sanome's MEMORI Platform Supports Earlier Detection of Hospital-Acquired Infections in NHS Trusts
- CMR Surgical Gains FDA Clearance for Versius Plus, Eyes US Market Entry in 2026
- Edwards gains FDA approval for Sapien M3 mitral valve replacement system
- FDA issues Class I recall for Medtronic heart vent catheters
- ZOLL launches next-generation LifeVest wearable cardioverter defibrillator in the US
- Shape Memory Medical secures EU MDR CE mark for embolisation plugs
- MARAbio introduces the MAR-Autism Test to identify maternal autoantibodies linked to autism
- XCath Develops Robotic System to Improve Brain Aneurysm and Stroke Care
