(An Autonomous Body Recognized by Ministry of Commerce & Industry, Government of India)
Competency based placement focussed Education | Training | Research | Consultancy
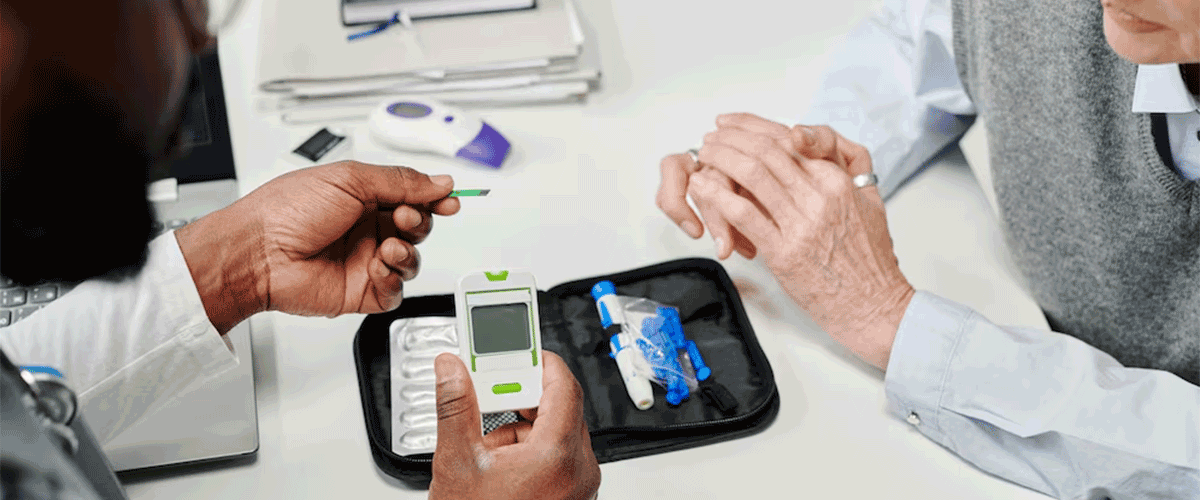
FDA Warns Dexcom Over G7 CGM Design Change
The FDA issued a warning letter to Dexcom over a design change affecting its G6 and G7 continuous glucose monitors (CGMs). The issue involves a new component in the resistance layer, which led to reduced accuracy. Dexcom has ceased distribution of affected G7 sensors but has yet to address impacted G6 sensors.
The design change, involving a chemical compound used in sensor wires, was made to diversify Dexcom’s supply chain. After FDA scrutiny, the company reverted to its original supplier. The FDA flagged risks for insulin dosing decisions and monitoring issues with glucose and acetaminophen concentrations.
Despite the warning, Dexcom does not expect delays in future FDA approvals, including its 15-day G7 CGM, set for launch in late 2024. There’s no recall yet, but the FDA continues monitoring compliance.
02-04-2025
📰 Recent News
- Union Health Minister Releases Indian Pharmacopoeia 2026, Strengthening Drug Quality Standards
- Valneva and Serum Institute End License Agreement for Chikungunya Vaccine
- NPPA Clears Serum Institute to Stop Two Tetanus Toxoid Pack Sizes
- DoP Extends PLI Application Deadline for Meropenem and Ritonavir
- India Bans High-Dose Nimesulide Oral Formulations Over Safety Concerns
- India Proposes Prescription-Only Sale of Cough Syrups After Child Deaths
- FDA Publishes Revised Draft Guideline on Medical Gases
- FDA Issues Warning Letter to Cdymax India Pharma Over OOS and CAPA Failures
- FDA Highlights Data Integrity Risks of Excel in GMP Environments
- ICH Moves Toward Harmonised Biosimilar Development Guidelines
