(An Autonomous Body Recognized by Ministry of Commerce & Industry, Government of India)
Competency based placement focussed Education | Training | Research | Consultancy
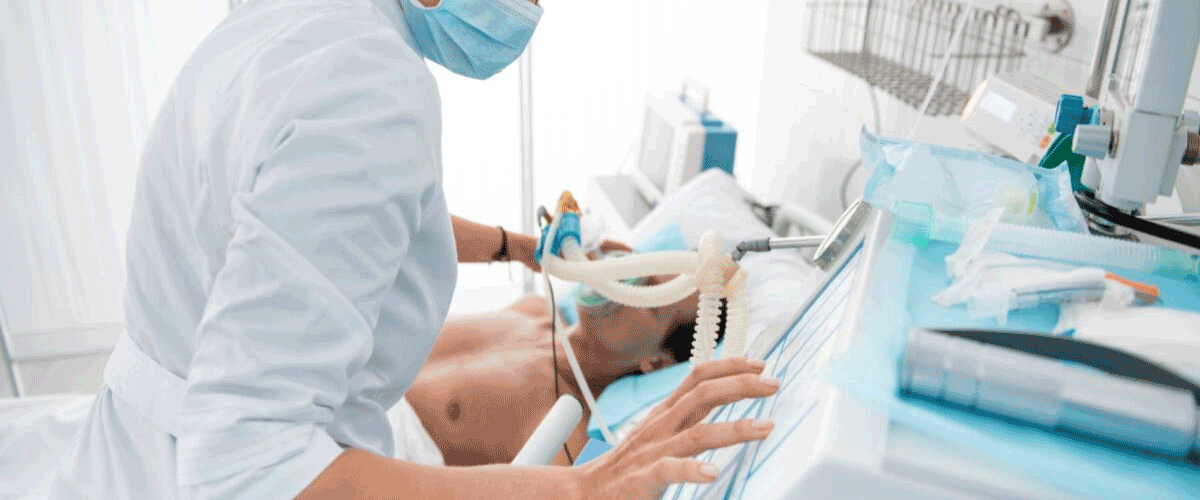
FDA Flags Draeger Recall After Faulty Filters Linked to Injuries
The U.S. Food and Drug Administration (FDA) has issued a Class I recall, its most serious classification, for millions of Draeger’s SafeStar and TwinStar mechanical filters used in anesthesia and mechanical ventilation. The filters, manufactured at Draeger’s headquarters in Lübeck, Germany, were found to produce misleading carbon dioxide readings, potentially leading clinicians to deliver unnecessary or harmful treatments.
The recall, initiated in June and updated with a customer notice on July 22, affects four filter models totaling nearly 12 million devices. The FDA confirmed that serious injuries have been reported due to inaccurate capnography readings but did not disclose the number of cases. No deaths have been reported.
Draeger has instructed hospitals to stop using the affected filters immediately, remove them from stock, and arrange for returns and replacements. The devices are commonly used during surgery and in intensive care units.
16-08-2025
📰 Recent News
- KORU Medical Seeks FDA Clearance to Expand FreedomEDGE into Oncology Infusions
- SleepRes Secures FDA Clearance for Adaptive KPAP Device to Treat Sleep Apnoea
- Sanome's MEMORI Platform Supports Earlier Detection of Hospital-Acquired Infections in NHS Trusts
- CMR Surgical Gains FDA Clearance for Versius Plus, Eyes US Market Entry in 2026
- Edwards gains FDA approval for Sapien M3 mitral valve replacement system
- FDA issues Class I recall for Medtronic heart vent catheters
- ZOLL launches next-generation LifeVest wearable cardioverter defibrillator in the US
- Shape Memory Medical secures EU MDR CE mark for embolisation plugs
- MARAbio introduces the MAR-Autism Test to identify maternal autoantibodies linked to autism
- XCath Develops Robotic System to Improve Brain Aneurysm and Stroke Care
