(An Autonomous Body Recognized by Ministry of Commerce & Industry, Government of India)
Competency based placement focussed Education | Training | Research | Consultancy
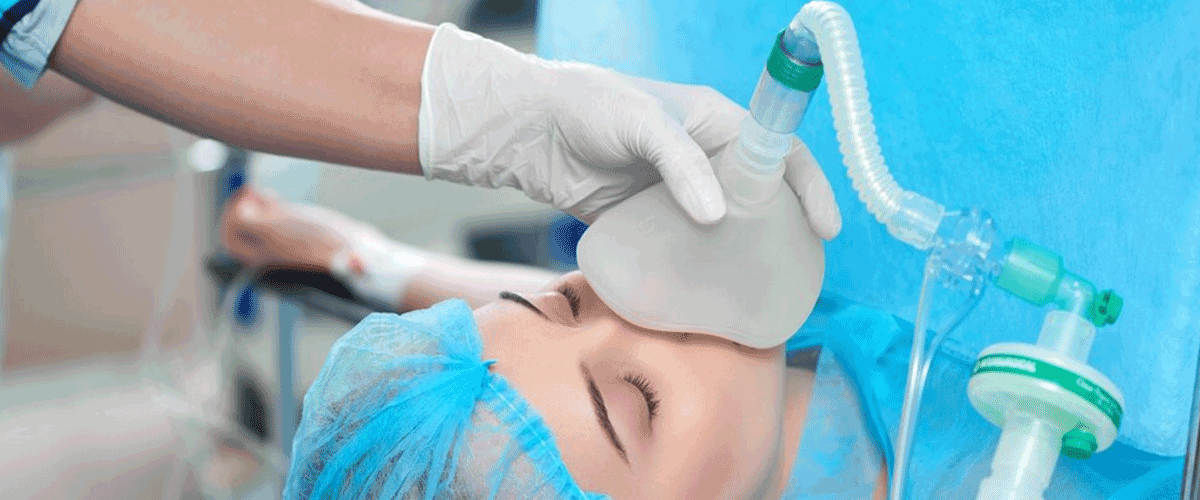
Indian hospitals effort to decrease sepsis incidents as US FDA norms stresses on close patient observation
Indian hospitals are intensifying efforts to reduce sepsis-related incidents, aligning with global standards set by the US FDA. Sepsis, a leading cause of hospital deaths, results from bacterial, mycobacterial, fungal, and viral infections. The US FDA emphasizes early detection and strict patient monitoring to improve survival rates.
The FDA’s updated guidance on donor eligibility and screening for sepsis aims to curb disease transmission through human cells and tissues. Victoria Hospital, Bengaluru, highlights the need for detailed monitoring, especially for economically disadvantaged patients with limited healthcare access. Measures include strict hand hygiene, sterile procedures, and early symptom recognition.
Medical students at Kempegowda Institute of Medical Sciences welcome the guidelines for identifying sepsis syndromes and systemic infections. While bacterial infections remain the most common cause, survivors face a higher risk of recurrence. Experts stress the importance of enhanced screening and monitoring to reduce sepsis-related mortality.
22-03-2025
📰 Recent News
- 2026 Emerges as Turning Point for Non-Invasive Drug Delivery in Pharma and MedTech
- Technology Integration Transforms Medical Transportation for Chronic Care in India
- Technology-Driven Innovations Reshape India’s Eye Care Ecosystem
- Xortx Underscores Genetic Evidence for Xanthine Oxidase Targeting and Announces Board Update
- Strengthening Regional Healthcare: A Priority for India’s Future
- Cybersecurity as a Core Pillar of Modern Hospital Operations
- Indian Healthcare in 2026: Mental Wellbeing and Technology Take Centre Stage
- Early Action Critical to Prevent a Human Bird Flu Outbreak, Study Warns
- The Trump administration rolls back Biden-era health IT rules, including AI ‘model card’ requirements.
- Belgium Pledges €8 Million to WHO to Boost Global Access to Health Technologies
