(An Autonomous Body Recognized by Ministry of Commerce & Industry, Government of India)
Competency based placement focussed Education | Training | Research | Consultancy
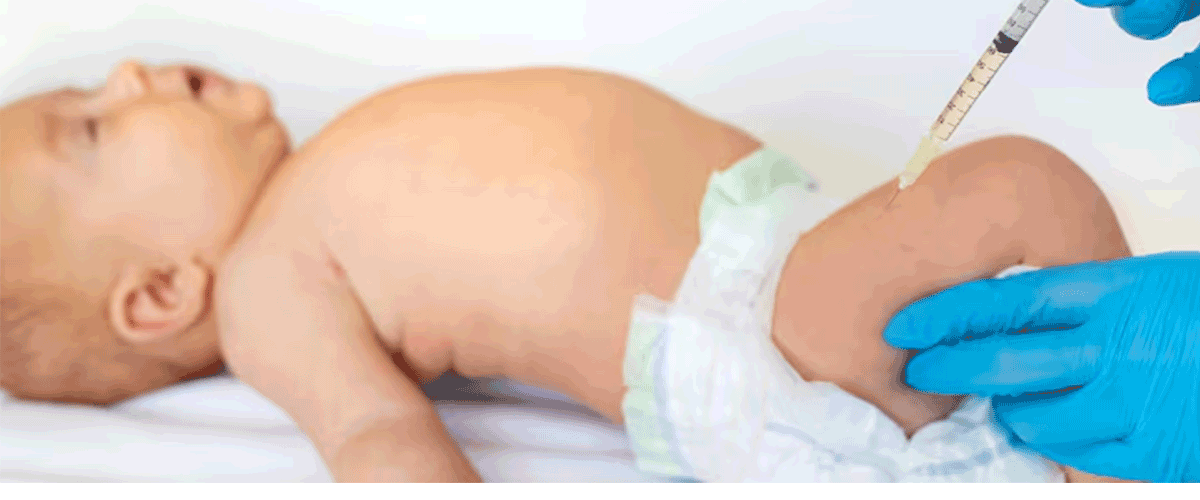
ACIP Postpones Vote on Delaying Newborn Hepatitis B Vaccine
The Advisory Committee on Immunization Practices (ACIP) postponed a planned vote on delaying the first hepatitis B vaccine dose for certain newborns, creating uncertainty over the childhood immunization schedule. The committee considered whether infants born to mothers who tested negative for hepatitis B could safely defer vaccination for at least one month.
Eleven ACIP members voted to table the decision indefinitely, citing unresolved questions on safety, effectiveness, and timing, while one member opposed tabling. Some panelists raised concerns about prenatal testing gaps and the risks of infants not receiving timely protection. The hepatitis B vaccine, recommended within 24 hours of birth and over 90% effective, prevents long-term liver disease, including cirrhosis and cancer.
Manufacturers Sanofi and Merck urged ACIP to maintain current guidance. With the vote postponed, it remains unclear when the committee will revisit the recommendation; a tentative meeting is scheduled for October.
21-09-2025
📰 Recent News
- 2026 Emerges as Turning Point for Non-Invasive Drug Delivery in Pharma and MedTech
- Technology Integration Transforms Medical Transportation for Chronic Care in India
- Technology-Driven Innovations Reshape India’s Eye Care Ecosystem
- Xortx Underscores Genetic Evidence for Xanthine Oxidase Targeting and Announces Board Update
- Strengthening Regional Healthcare: A Priority for India’s Future
- Cybersecurity as a Core Pillar of Modern Hospital Operations
- Indian Healthcare in 2026: Mental Wellbeing and Technology Take Centre Stage
- Early Action Critical to Prevent a Human Bird Flu Outbreak, Study Warns
- The Trump administration rolls back Biden-era health IT rules, including AI ‘model card’ requirements.
- Belgium Pledges €8 Million to WHO to Boost Global Access to Health Technologies
